Easy to learn, easy to use
OpenLab CDS
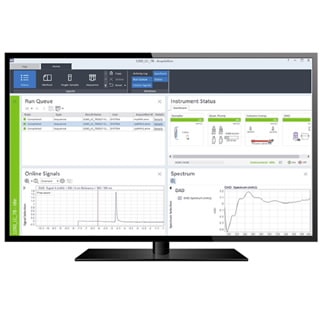
Built-in tools provide time-saving steps in the analysis, interpretation, and reporting workflows while technical controls ensure work quality, effective records management, and enhanced data security. OpenLab CDS is ideal for analytical labs that need the highest level of data integrity.
- Chromatography Data Systems
Request a Quote
Product Details
- Multi-vendor instrument control – Get the most comprehensive Agilent and non-Agilent instrument control of your LC, GC, LC/MS, GC/MS systems.
- Data integrity and compliance – Meet data integrity standards using built-in technical controls to securely acquire, process, report, and store data in laboratories that must follow the compliance guidelines of FDA 21 CFR Part 11, EU Annex 11, GAMP5 as well as ISO/IEC 17025 and EPA’s 40 CFR Part 160.
- Custom calculations and reporting – Automate calculations and reduce manual tasks to ensure quality and accuracy. Intuitive functionality makes report generation fast and easy.
- Enhanced data analysis – Speed data review and interpretation and perform basic and complex analysis to quickly identify key information.
- Application-specific software add-ons – Extend functionality or add additional capabilities with software add-ons such as Sample Scheduler, MatchCompare, Deconvolution, GPC, and ADFExport.
- Scalable architecture – Improve lab operations through centralization and get better access to instruments and information with a client/server configuration.
- On-demand learning – Onboard new staff quickly and effectively with a wide variety of learning tools to improve knowledge and performance.
- Automated software migration tools – Preserve result sets, methods, and user and instrument information from legacy ChemStation, ChemStation Edition, EZChrom Edition, and Galaxie CDS.
- Automated qualification tool – Perform software qualification and verification tasks to ensure your software is operating as intended.
- Flexible, remote access – Complete sample processing, review, and approval securely from home with virtualization, networking, and cloud solutions that make remote work simpler, safer, and more productive.
| Current Software Version |
|
| Deployment Model |
|
| Selectable Languages |
|
| Software Delivery |
|
| Supported Data Management |
|
| Supported Instruments |
|
| Supported Software Add-Ons |
|
| Techniques |
|
Videos
Fast, flexible, and reliable report generation
Reduce reporting time and ensure your results are clear and meaningful, and your calculations are always correct.
Efficient data analysis
Analyze and interpret data quickly by presenting information visually with visualization and peak assessment tools.
Support for Mass Spectrometry
Acquire, process, and report mass spectrometry data to meet your requirements and complete your workflow.
Automate data migration
Easily migrate your ChemStation or EZChrom data, methods, instrument information, and user roles and privileges.
Automate software verification with Test Services
Performs software verification tasks to confirm that the software is operating as intended.
Effective onboarding for lasting success
Train new staff quickly and get the most out of your software with self-guided learning tools.
- Key Literature
-
The Business Value of the Connected Lab: OpenLab CDS Client-Server
This white paper examines the business value of transitioning from isolated workstations to client-server architecture in analytical laboratories. Through OpenLab CDS Client-Server implementation, labs achieve enhanced operational efficiency, improved data security, and lower total costs. Case studies demonstrate 40-50% productivity gains, with ROI analysis showing benefits for labs operating three or more instruments. The paper provides practical implementation strategies and addresses key migration considerations.
- White Papers
- English
- 24 Jan 2025
- 472.79 KB
Purify with Ease Using Mass-Based Fraction Collection with InfinityLab Pro iQ and OpenLab CDS
The Agilent 1290 Infinity II Preparative LC/MSD System, InfinityLab Pro iQ Plus single quadrupole mass spectrometer (MS), combined with Agilent OpenLab CDS software, brings mass-based fraction collection into routine workflows with ease. This application note demonstrates the process applied to an elderberry extract targeting flavone glycosides.
- Application Notes
- English
- 01 Oct 2025
- 766.51 KB
- Application Notes
- Article Reprints
- Brochures
- Case Studies
- Flyers
- Posters
- Technical Overviews
- White Papers
- FAQs
-
What’s new in the latest release of OpenLab CDS?
Learn how new features and enhancements in OpenLab CDS version 2.8 help optimize workflows across energy, chemical, and pharmaceutical labs. This includes new calibration features to save time and reduce errors, enhanced technical controls for data integrity, and improved precision in peak integration.
What are the system requirements for OpenLab workstations?
See minimum requirements for Agilent OpenLab workstations including PC specifications, network specifications, and operating systems.
Can OpenLab CDS be deployed in the cloud?
Labs can now leverage cloud technology when deploying OpenLab CDS so they can scale quickly and better protect their vital data. We have supported deployments of OpenLab CDS and ECM XT on Amazon Web Services and Microsoft Azure.
Can I upgrade to the newest version of Windows when it is released by Microsoft?
We recommend waiting to upgrade to new versions of Windows until we've fully tested the new version.
Is OpenLab CDS supported with an LTSC version of Microsoft Windows?
While OpenLab CDS is mainly used with standard SASC (semi-annual service channel) versions of Windows included with most personal computers, Agilent does test and support the LTSC (long-term service channel) version.
What is a feature pack and what does it include?
Find detailed information about OpenLab CDS feature packs, including their contents and the naming conventions used.
What ASTM test methods are supported in OpenLab CDS?
See list of supported ASTM methods in OpenLab CDS version 2.7 and above.
How to Generate the License File in SubscribeNet
See the process for generating a permanent license file in SubscribeNet for OpenLab CDS software products.
Register an OpenLab CDS License Authorization Code in SubscribeNet
Learn how to create a SubscribeNet account and register OpenLab CDS license authorization codes.
- Quick Reference Guides
- Revision Tables
- Status Bulletins
- User Manuals
Didn't find what you need? Contact our support team.
The newest release of OpenLab CDS is here!
OpenLab CDS now fully supports the Agilent Infinity III LC series, enabling barcode-centric workflows, support for LC solvent level sensing and solvent prediction, and seamless integration with Sample Linking Software.
- 01 May 2025
Stay in the know with the latest data integrity trends
Data Integrity Insights is a collection of articles providing tips, best practices, and real software solutions for addressing common data integrity challenges. Explore topics around Analysis & Reporting, Inspection Readiness, Cloud, and more.
- 25 Apr 2022
Agilent Offers GC Image GCxGC Software for Comprehensive 2D-GC
GC Image GCxGC software is now available directly from Agilent. Chromatographers can use the unique cryogen-free Agilent Capillary Flow Technology reversed flow modulator to switch between columns and GC Image GCxGC software to visualize, analyze, and process data. This powerful combination provides a complete single-vendor solution for comprehensive two-dimensional GC.
- 04 Dec 2024
Three Winning Arguments to Get Management Approval for a CDS Upgrade
Getting management approval for a chromatography data system (CDS) upgrade is not always easy. Learn winning arguments to get approval for a CDS upgrade in your lab.
OpenLab CDS Webinar Series
Free webinars offering high level training of the latest OpenLAB CDS platform OpenLAB CDS 2.x provides the most complete instrument control of Agilent LC, GC, CE, CE/MS, and LC/MS instruments systems you have now, with limited instrument control of other vendors’ systems.
Data Integrity Webinars & Podcasts
Series of webinars to help your lab prepare for regulatory examination. Stay informed about the latest FDA and global enforcement trends and learn the strategies you can use to stay compliant.
Damian Connolly
Senior Analytical Scientist, APC
I would definitely recommend OpenLab CDS to my colleagues. It offers seamless integration of new equipment into an ever-expanding laboratory.
View MoreKeith Wilkins, PhD
Lead Analytical Chemist, Chemours
The flexibility to adjust reports with custom calculations and alerts is unique to the reporting solution.
View MoreGianfranco Brendolan
Owner CEO, SGB CONSULTORIA QUIMICA LTDA
[OpenLab CDS] is a robust and easy to use system. Excellent in reporting.
View More