PD-L1 IHC 22C3 pharmDx - Product Page

- PD-L1 IHC 22C3 pharmDx is a clinical trial-proven companion diagnostic
CE-IVD—marked to cover four CDx indications - PD-L1 IHC 22C3 pharmDx is the only assay used to assess PD‑L1 across pivotal KEYTRUDA® (pembrolizumab) clinical trials and the PD‑L1 assay used in the pivotal LIBTAYO® (cemiplimab) clinical trial (Study 1624) in advanced NSCLC
High specificity for accurate clinical results
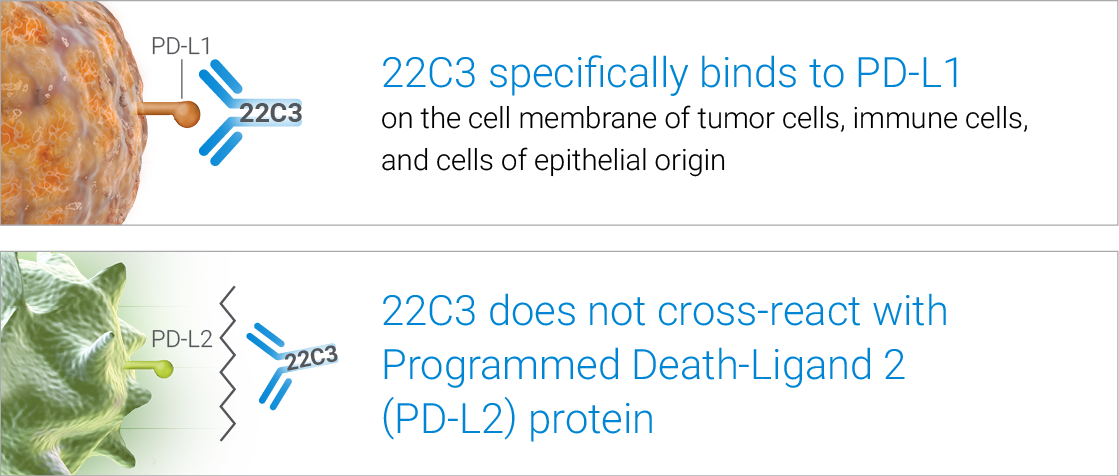
High sensitivity for precise PD‑L1 detection
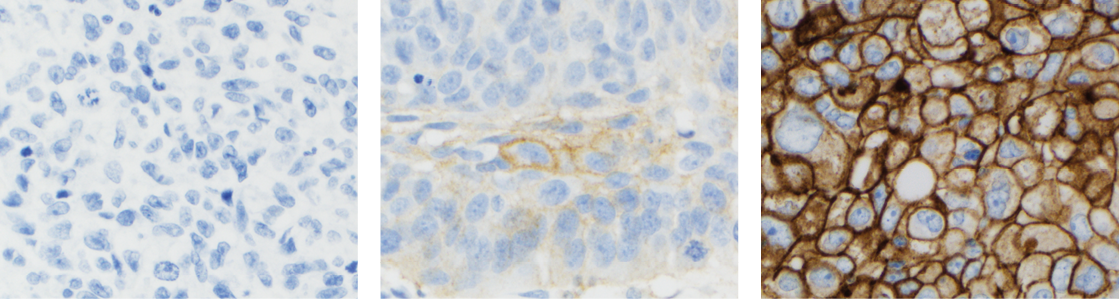
Assessment of PD-L1 expression in NSCLC demonstrated staining across the dynamic range of TPS
0–100% and 0–3+ staining intensities


- Agilent performed rigorous repeatability testing to ensure reliable results
- 100% overall agreement across inter-instrument, inter-operator, inter-day, and intra-run tests*
- Reproducibility testing performed at three external testing sites demonstrated > 85% overall agreement across all tests*
- Inter-site, intra-site, inter-observer, and intra-observer tests were all performed
- PD-L1 IHC 22C3 pharmDx comes pre-validated, including validated scoring guidelines and on‑site product demonstrations
- All necessary components for a complete staining run are validated together and included in each kit
Agilent supports accurate PD‑L1 scoring and interpretation with a full suite of training resources to increase confidence in evaluating PD‑L1 expression using Combined Positive Score (CPS) or Tumor Proportion Score (TPS)
- PD-L1 IHC 22C3 pharmDx is a standardized IHC assay with all necessary components for 50 tests in one kit
- PD-L1 IHC 22C3 pharmDx is designed for an automated staining procedure for enhanced operational efficiency
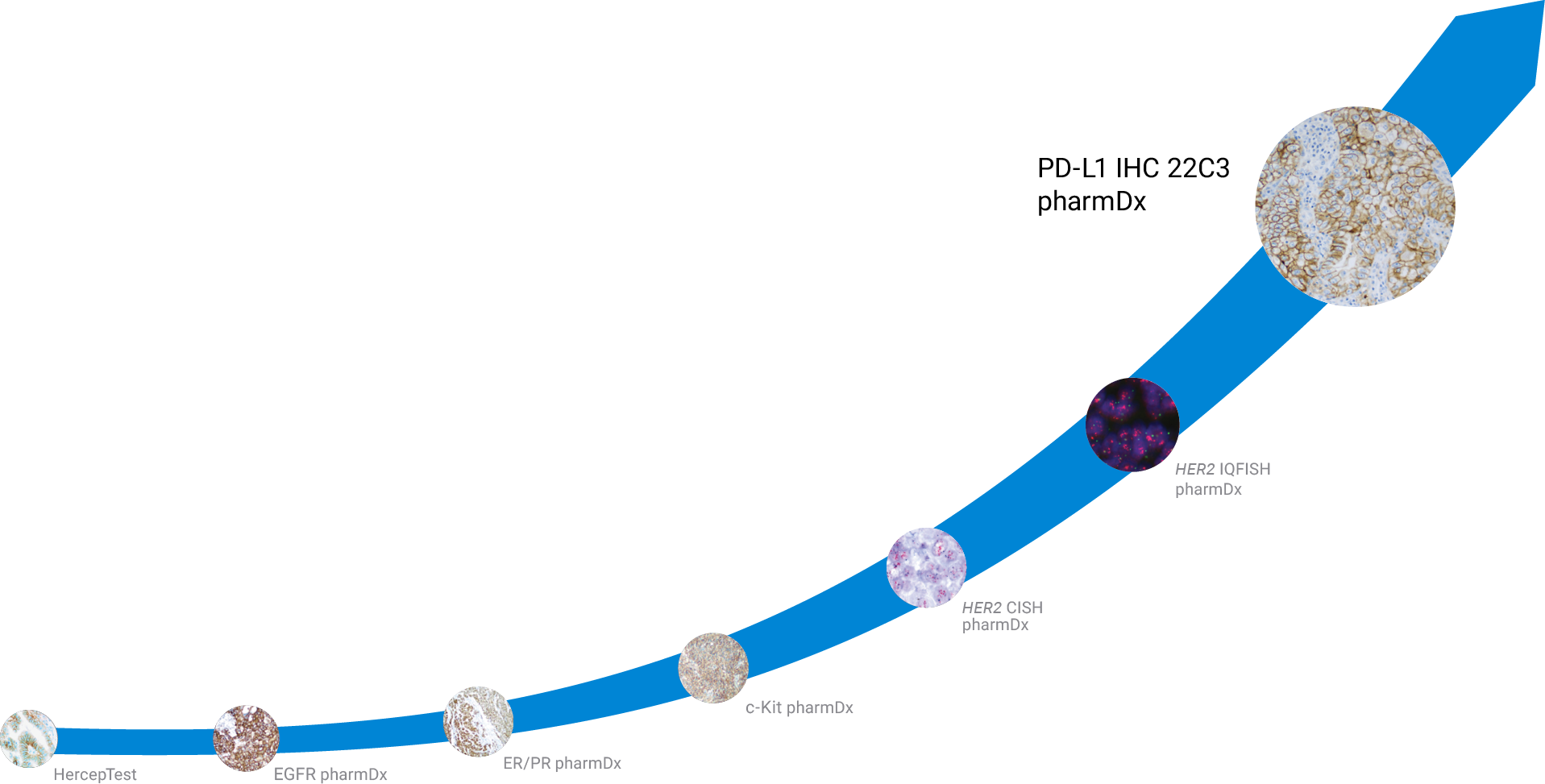
Agilent has a legacy of pioneering diagnostic assay development to support the development, validation, and launch of landmark therapies
Intended Use
For in vitro diagnostic use.
PD-L1 IHC 22C3 pharmDx is a qualitative immunohistochemical assay using monoclonal mouse anti-PD-L1, Clone 22C3, intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC), urothelial carcinoma, head and neck squamous cell carcinoma (HNSCC), and melanoma tissues using EnVision FLEX visualization system on Autostainer Link 48.
PD-L1 protein expression in NSCLC is determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity.
PD-L1 protein expression in urothelial carcinoma is determined by using Combined Positive Score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100.
PD-L1 protein expression in HNSCC is determined by using CPS and/or TPS.
PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying patients for treatment with the therapies for the indications listed in Table 1.
Table 1: PD-L1 IHC 22C3 pharmDx companion diagnostic indications, PD-L1 expression levels, and therapies
| Tumor Indication | PD-L1 Expression Level | Therapy |
| NSCLC | TPS ≥ 1% TPS ≥ 50% |
|
| Urothelial Carcinoma | CPS ≥ 10 | KEYTRUDA®* |
| HNSCC | CPS ≥ 1 TPS ≥ 50% |
|
| NSCLC | TPS ≥ 50% | LIBTAYO®** |
* See the KEYTRUDA® product label for PD-L1 expression cutoff values and specific clinical circumstances guiding therapy.
** See the LIBTAYO® product label for specific clinical circumstances guiding therapy.
KEYTRUDA is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
LIBTAYO is a registered trademark of Regeneron Pharmaceuticals, Inc.
References: 1. PD-L1 IHC 22C3 pharmDx [Instructions for Use]. Santa Clara, CA: Agilent Technologies, Inc.; 2021. 2. Keytruda [Summary of Product Characteristics]. European Medicines Agency; 2020. 3. Libtayo [Summary of Product Characteristics]. European Medicines Agency; 2021. 4. Garon, E. B.; Rizvi, N. A.; Hui, R.; Leighl, N.; Balmanoukian, A. S.; Eder, J. P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; Carcereny, E.; Ahn, M.-J.; Felip, E.; Lee, J.-S.; Hellmann, M. D.; Hamid, O.; Goldman, J. W.; Soria, J.-C.; Dolled-Filhart, M.; Rutledge, R. Z.; Zhang, J.; Lunceford, J. K.; Rangwala, R.; Lubiniecki, G. M.; Roach, C.; Emancipator, K.; Gandhi, L. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372 (21), 2018–2028. 5. Herbst, R. S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J. L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C. D.; Ahn, M.-J.; Majem, M.; Fidler, M. J.; de Castro, G. Jr.; Garrido, M.; Lubiniecki, G. M.; Shentu, Y.; Im, E.; Dolled-Filhart, M.; Garon, E. B. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016, 387 (10027), 1540–1550. 6. Reck, M.; Rodríguez-Abreu, D.; Robinson, A. G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; O'Brien, M.; Rao, S.; Hotta, K.; Leiby, M. A.; Lubiniecki, G. M.; Shentu, Y.; Rangwala, R.; Brahmer, J. R. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375 (19), 1823–1833. 7. Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H. M.; Cicin, I.; Bentsion, D.; Gladkov, O.; Clingan, P.; Sriuranpong, V.; Rizvi, N.; Gao, B.; Li, S.; Lee, S.; McGuire, K.; Chen, C.-I.; Makharadze, T.; Paydas, S.; Nechaeva, M.; Seebach, F.; Weinreich, D. M.; Yancopoulos, G. D.; Gullo, G.; Lowy, I.; Rietschel, P. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397 (10274), 592–604. 8. Roach, C.; Zhang, N.; Corigliano, E.; Jansson, M.; Toland, G.; Ponto, G.; Dolled-Filhart, M.; Emancipator, K.; Stanforth, D.; Kulangara, K. Development of a Companion Diagnostic PD-L1 Immunohistochemistry Assay for Pembrolizumab Therapy in Non–Small-cell Lung Cancer Appl. Immunohistochem. Mol. Morphol. 2016, 24 (6), 392–397.
For countries outside of the European Union, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.
For countries outside of the European Union, see the local LIBTAYO product label for approved indications.
D66805/01 2021AUG04