PD-L1 IHC 22C3 pharmDx Overview

- With indications across a wide range of cancer types, oncologists are increasingly selecting patients for treatment with KEYTRUDA
- PD-L1 IHC 22C3 pharmDx is the clinically relevant option when considering patients for treatment with KEYTRUDA
High specificity for accurate clinical results
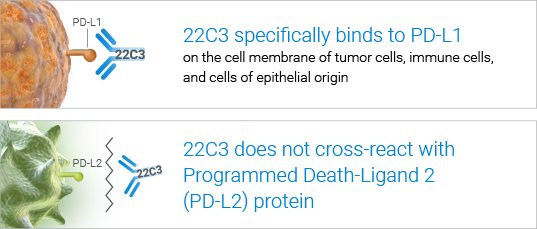
High sensitivity for precise PD-L1 detection
- Assessment of PD-L1 expression in NSCLC demonstrated staining across the dynamic range of TPS 0–100% and 0–3+ staining intensities
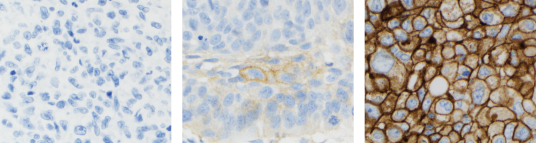
The stains above are of NSCLC specimens stained with PD-L1 IHC 22C3 pharmDx.

- Agilent performed rigorous repeatability testing to ensure reliable results
- 100% overall agreement across inter-instrument, inter-operator, inter-day, and intra-run tests*

- Reproducibility testing performed at three external testing sites demonstrated > 85% overall agreement across all tests*
- Inter-site, intra-site, inter-observer, and intra-observer tests were all performed

- PD-L1 IHC 22C3 pharmDx comes pre-validated, including validated scoring guidelines and on-site product demonstrations
- All necessary components for a complete staining run are validated together and included in each kit
* Overall agreement is based on average negative and average positive agreements. Tests were performed using NSCLC tissue
- PD-L1 IHC 22C3 pharmDx is a standardized IHC assay with all necessary components for 50 tests in one kit
- Designed for use on Autostainer Link 48, which comes preprogrammed with a validated staining protocol in the DakoLink software

Intended Use
For in vitro diagnostic use.
PD-L1 IHC 22C3 pharmDx is a qualitative immunohistochemical assay using monoclonal mouse anti-PD-L1, Clone 22C3 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC), esophageal squamous cell carcinoma (ESCC), cervical cancer, head and neck squamous cell carcinoma (HNSCC), and triple-negative breast cancer (TNBC) tissues using EnVision FLEX visualization system on Autostainer Link 48.
PD-L1 protein expression in NSCLC is determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity.
PD-L1 protein expression in ESCC, cervical cancer, HNSCC, and TNBC is determined by using Combined Positive Score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100.
Companion Diagnostic Indications
| Tumor Indication | PD-L1 Expression Level | Intended Use |
| NSCLC | TPS ≥ 1% | PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying NSCLC patients for treatment with KEYTRUDA® (pembrolizumab).** |
| ESCC | CPS ≥ 10 | PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying ESCC patients for treatment with KEYTRUDA® (pembrolizumab). |
| Cervical Cancer | CPS ≥ 1 | PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying cervical cancer patients for treatment with KEYTRUDA® (pembrolizumab). |
| HNSCC | CPS ≥ 1 | PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying HNSCC patients for treatment with KEYTRUDA® (pembrolizumab).** |
| TNBC | CPS ≥ 10 | PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying TNBC patients for treatment with KEYTRUDA® (pembrolizumab). |
** See the KEYTRUDA® product label for specific clinical circumstances guiding PD-L1 testing.
KEYTRUDA is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
References: 1. PD-L1 IHC 22C3 pharmDx [Instructions for Use]. Santa Clara, CA: Agilent Technologies, Inc.; 2022. 2. Keytruda [package insert]. 3. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. 4. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. 5. Reck M, Rodríguez-abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. 6. Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392-397.
For countries outside of the United States, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.
D53208_06