

- Broad Utility: Evidence based PD-L1 testing across multiple cancer types
- Clinically relevant PD-L1 results linked to clinical outcomes
- Unrivaled quality and reliability
- Standardized and fully validated kit
- Integrate PD-L1 IHC 28-8 pharmDx without changing staining lab workflow
- Ready-to-use reagents and cell line controls optimized for Autostainer Link 48
- Pre-programmed, validated protocol
Non-small cell lung cancer (NSCLC)
Click here to download Manual and Brochure
Non-squamous non-small cell lung cancer (nsNSCLC)
Click here to download Manual and Brochure
Squamous Cell Carcinoma of the head and neck (SCCHN)
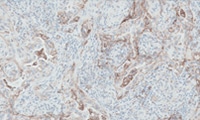
Scoring Guidelines
Read about the interpretation of PD-L1 IHC 28-8 pharmDx staining results.
Interpretation of NSCLC staining results
Interpretation of non-squamous NSCLC staining results
Interpretation of SCCHN staining results
Interpretation of UC staining results
Sign up now for new interactive online PD-L1 interpretation program
PD-L1 IHC 28-8 pharmDx is a complete kit with reagents sufficient for 50 tests (50 slides incubated with primary antibody to PD-L1 and 50 slides incubated with the corresponding negative control reagent) and 15 Control Slides for use on Autostainer Link 48.

- EnVision FLEX Target Retrieval Solution, Low pH, 50x
- Peroxidase-Blocking Reagent
- Monoclonal Rabbit Anti-PD-L1, Clone 28-8
- Negative Control Reagent
- PD-L1 IHC 28-8 pharmDx Rabbit LINKER
- Visualization Reagent-HRP
- DAB+ Substrate Buffer
- DAB+ Chromogen
- DAB Enhancer
- Control Slides
|
Product |
Code |
|---|---|
|
SK005 |
|
|
Required but not included in the kit: Autostainer Link 48 EnVision FLEX Wash Buffer, 20x EnVision FLEX Hematoxylin (Link) PT Link PT Link Rinse Station |
AS480 K8007 K8008 PT101/PT200 PT109 |
Intended Use
For in vitro diagnostic use.
PD-L1 IHC 28-8 pharmDx is a qualitative immunohistochemical assay using Monoclonal Rabbit Anti-PD-L1, Clone 28-8 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC), squamous cell carcinoma of the head and neck (SCCHN), and urothelial carcinoma (UC) tissues using EnVision FLEX visualization system on Autostainer Link 48.
PD-L1 protein expression is defined as the percentage of evaluable tumor cells exhibiting partial or complete membrane staining at any intensity.
Companion Diagnostic Indication
|
Tumor Indication |
PD-L1 Expression Clinical |
Intended Use |
|
NSCLC |
≥ 1% tumor cell expression |
PD-L1 IHC 28-8 pharmDx is indicated as an aid in identifying NSCLC patients for treatment with OPDIVO® (nivolumab) in combination with YERVOY® (ipilimumab). |
PD-L1 expression (≥ 1% or ≥ 5% or ≥ 10% tumor cell expression), as detected by PD-L1 IHC 28-8 pharmDx in non-squamous NSCLC may be associated with enhanced survival from OPDIVO®.
PD-L1 expression (≥ 1% tumor cell expression), as detected by PD-L1 IHC 28-8 pharmDx in SCCHN may be associated with enhanced survival from OPDIVO®.
PD-L1 expression (≥ 1% tumor cell expression), as detected by PD-L1 IHC 28-8 pharmDx in UC may be associated with enhanced response rate from OPDIVO®.
See the OPDIVO® and YERVOY® product labels for specific clinical circumstances guiding PD-L1 testing.
References
1. PD-L1 IHC 28-8 pharmDx Instructions for Use
2. CHECKMATE-227, CA209227
3. CHECKMATE-057, CA209057
4. CHECKMATE-141, CA209141
5. CHECKMATE-275, CA209275
