PD-L1 IHC 28-8 pharmDx for Urothelial Carcinoma


PD-L1 IHC 28-8 pharmDx for UC
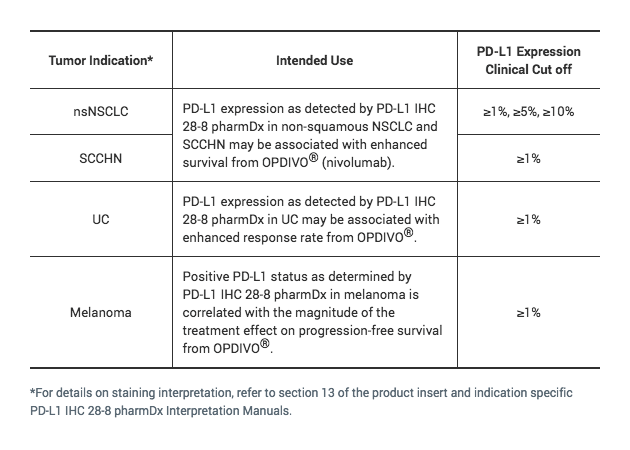
The only FDA-approved test for which PD-L1 expression in urothelial carcinoma may be associated with enhanced response rate from OPDIVO® (nivolumab)1
Demonstrated clinical results
Detection of PD-L1 expressing tumor cells in UC patient specimens may indicate an enhanced response rate benefit to OPDIVO (nivolumab) treatment for the patient.2
In study CHECKMATE-275, objective response rate (ORR) based on PD-L1 expression was evaluated using PD-L1 IHC 28-8 pharmDx and is summarized below. Median time to response was 1.9 months (range; 1.6-7.2).
Efficacy results for study CHECKMATE-2751
Confirmed ORR in all patients and the two PD-L1 subgroups are summarized in the table below.
| Tumor PD-L1 Expression | <1% | ≥1% | All Treated Subjects |
|---|---|---|---|
| Total No. of Subjects | N=146 | N=124 | N=270 |
| Confirmed Objective Response Rate No. of Subjects (95% CI) |
22 (9.7, 21.9) |
31 (17.7, 33.6) |
53 (15.1, 24.9) |
| Complete Response Rate No. of Subjects (% of Total in PD-L1 expression category) |
1 (0.7%) |
6 (4.8%) |
7 (2.6%) |
| Partial Response Rate No. of Subjects (% of Total in PD-L1 expression category) |
21 (14.4%) |
25 (20.2%) |
46 (17.0%) |
| Median Duration of Response* Months (range) |
7.6 mos. (3.7+, 12.0+) |
NE (1.9+, 12.0+) |
10.3 (1.9+, 12.0+) |
*Estimated from the Kaplan-Meier Curve
Clinical utility of PD-L1 IHC 28-8 pharmDx was evaluated in CHECKMATE-275, a phase II single arm clinical trial of nivolumab in subjects with metastatic or unresectable urothelial carcinoma or who have progressed or recurred following treatment with a platinum agent. 270 subjects were randomized to receive the drug at 63 sites in 11 countries. Major efficacy outcome measures included confirmed objective response rate (ORR) and duration of response (DOR).
Frequency of PD-L1 expression in samples from UC - CHECKMATE-2751
| Tumor PD-L1 Expression | Nivolumab (N=270) |
|---|---|
| ≥1% PD-L1 Expression Subjects | 124 (45.9%) |
| <1% PD-L1 Expression Subjects | 146 (54.1%) |
Baseline UC specimen origin - CHECKMATE-2751
|
Non-bladder UC 27%(73/270) |
Visceral metastases 84%(227/270) |
- 27% of nivolumab treated patients had non-bladder urothelial carcinoma
- Regardless of tumor site, 84% of all treated patients presented with visceral metastases at baseline
Robust performance
PD-L1 IHC 28-8 pharmDx is fully validated for analytical performance, having met stringent acceptance criteria for ultimate quality results.
| Selected analytical validation parameters |
Results for UC |
|---|---|
| Analytical specificity |
|
| Sensitivity |
|
| Repeatability |
|
| External reproducibility |
|
| ANA = Average Negative Agreement | APA = Average Positive Agreement | OA = Overall Agreement | |
Order information
| Product | Code |
|---|---|
| PD-L1 IHC 28-8 pharmDx | SK005 |
| Required but not included in kit: | |
| Autostainer Link 48 | AS480 |
| EnVision FLEX Wash Buffer, 20x | K8007 |
| EnVision FLEX Hematoxylin (Link) | K8008 |
| PT Link | PT101/PT200 |
| PT Link rinse station | PT109 |
References
- Clinical Trial: CHECKMATE-275, CA209275
- PD-L1 IHC 28-8 pharmDx Instructions for Use