Access Agilent eNewsletter June 2016

Robust Agilent 6470 Triple Quadrupole LC/MS delivers confident quantification and streamlined workflows
Badr Astiphan, Agilent Marketing Manager—Quadrupole Mass Spectrometry
Whether you work in food testing or environmental analysis, drug development or clinical research, you must meet the requirements for stringent LC/MS quantification in the face of challenges such as time-consuming sample preparation, limited amounts of sample, complex matrices, and the need for efficient throughput.
The Agilent 6470 Triple Quadrupole LC/MS system provides excellent sensitivity, precision, and scan speed, to allow you to streamline analytical workflows. You can dilute or use less sample and still have confidence in the accuracy of your results. The system’s proven reliability means less maintenance and more uninterrupted laboratory productivity.
Robust quantification of target pharmaceuticals in dirty sample matrices
The Agilent 6470 delivers robust performance for a host of in vitro and in vivo assays to screen potential drug candidates for further development. In vivo identification and quantification of metabolites are specifically challenging because nonvolatile sample matrix components can accumulate in the ion source and ion transfer optics, which degrades performance over time and necessitates frequent cleaning.
The Agilent 6470 Triple Quadrupole LC/MS system reduces these maintenance requirements. Several key design innovations deliver sensitive, precise, and robust quantification of target analytes in heavy sample matrices over a wide linear dynamic range. These features include:
- Optimized ion optics and prefilter geometries, which increase ion transmission and minimize contamination
- A curved and tapered hexapole collision cell, which enables high-efficiency MS/MS fragmentation and transmission of ions
- An ion detector with a high-energy conversion dynode and low noise characteristics for efficient positive and negative ion detection across a wide m/z range
As one example, Agilent Application Note 5991-5953EN demonstrates the advantages of the Agilent 6470 Triple Quadrupole LC/MS coupled to an Agilent RapidFire 365 High-throughput MS System for the rapid and robust analysis of pharmaceuticals in spiked precipitated porcine plasma.
Figure 1 shows the peak area ratios for alprazolam and clozapine from the injection of 50 picograms of each analyte as a function of the RapidFire injection number. Stable peak area ratios were obtained over the 10,000 injections, clearly demonstrating robust analytical performance.
The innovative ion transfer optics design of the Agilent 6470 Triple Quadrupole LC/MS system minimizes the adverse effects of sample accumulation to provide a robust analytical platform for the ultra-high throughput analysis of target drug candidates in difficult precipitated plasma samples.
Reliable quantification of pesticides in food samples
Application Note 5991-6357EN describes use of the Agilent 6470 Triple Quadrupole LC/MS system for a UHPLC/MS/MS-based method for the screening and quantification of more than 250 pesticides and pesticide metabolites in food samples. This method leverages the:
- Increased chromatographic resolution of the Agilent 1290 Infinity Binary LC System.
- Versatile ionization capabilities of the Agilent Jet Stream ionization source.
- Innate sensitivity of the Agilent 6470 Triple Quadrupole LC/MS system.

Figure 2. Overlapped MRM chromatograms of more than 250 pesticides spiked into tea at the maximum residue limit (10 µg/kg) and diluted 1:10 with acetonitrile. Final concentrations were 0.2 ng/mL.
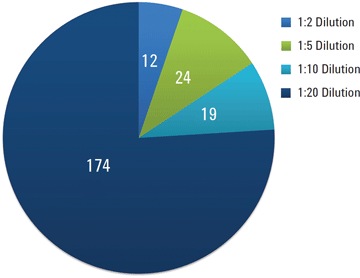
Figure 3. Number of pesticides detected in samples that were diluted to minimize matrix effects. Instrument sensitivity enabled reliable detection despite dilution.
The method was applied to the analysis of pesticide residues in complex food matrices. Figure 2 shows overlapped multiple reaction monitoring (MRM) chromatograms of more than 250 pesticides spiked into black tea. The instrument sensitivity permitted sample dilution prior to injection, which minimized ion suppression due to matrix effects and enabled more accurate quantification.
Increase lab productivity
Sample dilution improved method robustness and increased instrument uptime. We were able to quantify the majority of pesticides with acceptable recovery from 70 to 120 percent, based on a solvent calibration. The increased sensitivity of the 6470 Triple Quadrupole LC/MS system allowed the quantification of most of the targeted pesticides below the maximum residue limits (MRLs) specified by the European Commission.
Figure 3 shows detection rates of pesticides spiked into black tea extracts at 10 µg/kg and diluted with acetonitrile. We detected 174 pesticides at the 1:20 dilution level with an area RSD less than 20 percent. Additional compounds were detected at lower dilution levels (higher concentrations).
Streamline your workflows with the Agilent 6470 QQQ
These examples demonstrate that the Agilent 6470 Triple Quadrupole LC/MS system is our most robust triple quadrupole ever and enables streamlined workflows for pharmaceutical, food, environmental, and clinical research analyses.
To learn more about this technique read Agilent Application Note 5991-3344EN, which describes a high-throughput, two-minute analytical method for the quantification of four immunosuppressants in whole blood matrix using an Agilent 6460 or 6470 Triple Quadrupole LC/MS system. A simple protein precipitation followed by automated online sample cleanup minimized the matrix effect and ion suppression due to biological compounds present in blood.
Discover more today about this rock solid LC/MS.
For Research Use Only. Not for use in diagnostic procedures.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
All articles in this issue
 New Agilent 8900 ICP-QQQ delivers increased sensitivity, improved accuracy, and higher productivity
New Agilent 8900 ICP-QQQ delivers increased sensitivity, improved accuracy, and higher productivity New Agilent 1260 Infinity II LC system delivers higher level of operational efficiency
New Agilent 1260 Infinity II LC system delivers higher level of operational efficiency Robust Agilent 6470 Triple Quadrupole LC/MS delivers confident quantification and streamlined workflows
Robust Agilent 6470 Triple Quadrupole LC/MS delivers confident quantification and streamlined workflows Achieve accurate analysis of sulfur and nitrogen with the new and simplified Agilent 8355 SCD
Achieve accurate analysis of sulfur and nitrogen with the new and simplified Agilent 8355 SCD Agilent 1290 LC and 6550 iFunnel Q-TOF combine to provide fast, high-resolution peptide mapping of innovator and biosimilar mAbs
Agilent 1290 LC and 6550 iFunnel Q-TOF combine to provide fast, high-resolution peptide mapping of innovator and biosimilar mAbs Agilent collaborates with Academia—from investigating arsenic mysteries to understanding the complexity of biology
Agilent collaborates with Academia—from investigating arsenic mysteries to understanding the complexity of biology Excellent inertness for analysis of challenging polar compounds
Excellent inertness for analysis of challenging polar compounds Detection of semivolatile extractables and leachables (E&Ls) found in pharmaceutical products
Detection of semivolatile extractables and leachables (E&Ls) found in pharmaceutical products Agilent multi-omics solutions unravel the effects of low-level gamma radiation on rice seeds
Agilent multi-omics solutions unravel the effects of low-level gamma radiation on rice seeds Agilent comprehensive water screening solution reliably identifies wastewater contaminants of emerging concern:
Agilent comprehensive water screening solution reliably identifies wastewater contaminants of emerging concern:
A real-life application
Figure 1
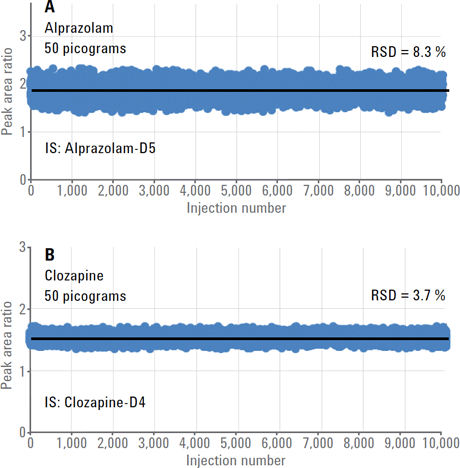
Consistent peak area ratios for (A) alprazolam and (B) clozapine over 10,000 injections.
Figure 2

Overlapped MRM chromatograms of more than 250 pesticides spiked into tea at the maximum residue limit (10 µg/kg) and diluted 1:10 with acetonitrile. Final concentrations were 0.2 ng/mL.
Figure 3

Number of pesticides detected in samples that were diluted to minimize matrix effects. Instrument sensitivity enabled reliable detection despite dilution.