Access Agilent eNewsletter June 2016
Agilent 1290 LC and 6550 iFunnel Q-TOF combine to provide fast, high-resolution peptide mapping of innovator and biosimilar mAbs
Ravinda Gudihal, Application Specialist, Agilent Technologies
Today, development of biosimilars continues to expand due to patent expirations on innovator drugs, especially in developing countries. Biosimilar monoclonal antibodies (mAbs) are replicas of licensed innovator products and are now considered one of the most important classes of biomolecules for the treatment of various cancers. In this article, we explore techniques and applications used for peptide mapping of biosimilar mAbs.
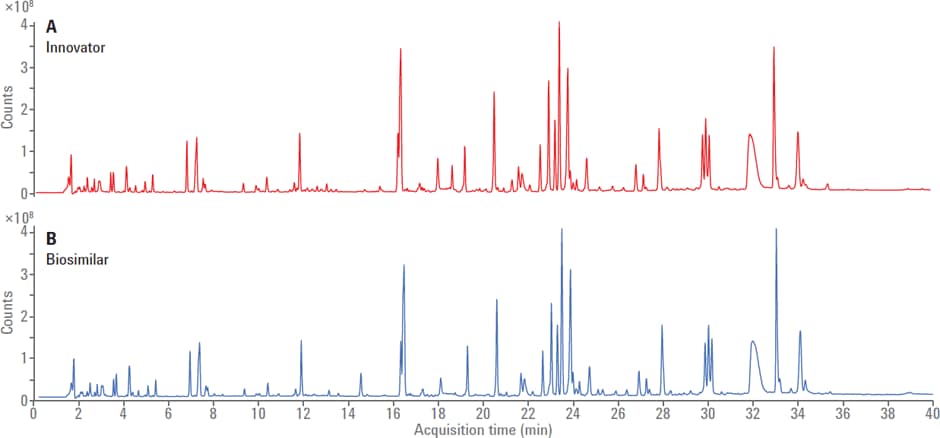
Figure 1. Total ion chromatogram (TIC) of trypsin-digested mAb obtained using LC/MS for (A) innovator and (B) biosimilar with an Agilent AdvanceBio peptide mapping column.
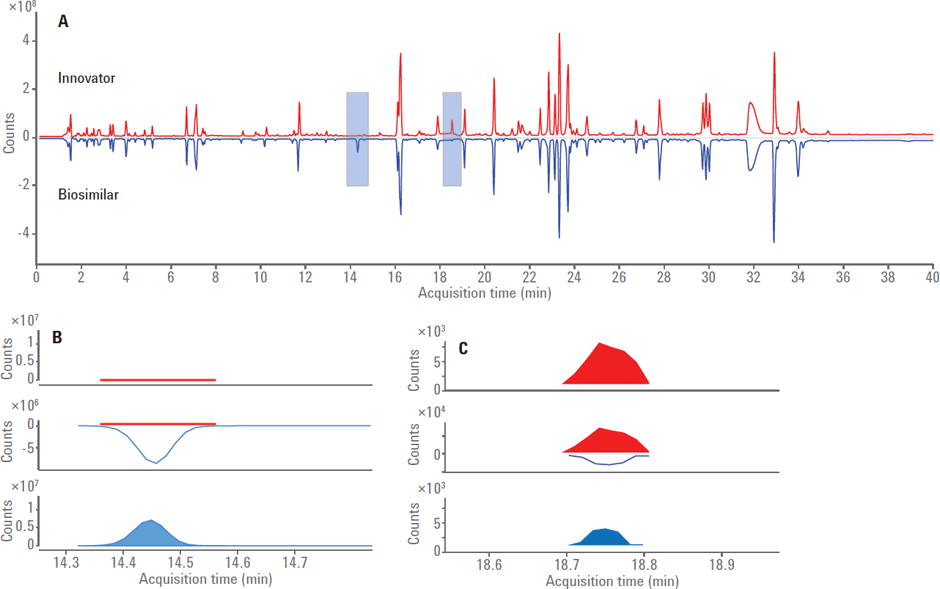
Figure 2. Agilent MassHunter BioConfirm Software Comparative Analysis feature.
Peptide mapping is critical in evaluating quality of antibodies
In the pharmaceutical industry, peptide mapping is a regularly employed technique for evaluating the quality of antibodies and is critical in showing the similarity in sequence and modifications between innovator and biosimilar pairs. Peptide mapping involves protease digestion of proteins/mAbs followed by LC/MS/MS analysis. To ensure the quality of biosimilar mAbs, and to show molecular similarity with the innovators, their amino acid sequence confirmation is of the utmost importance.
In one application, commercial Rituximab—used in the treatment of lymphomas, arthritis, and leukemia—innovator and biosimilar was subjected to trypsin digestion followed by peptide separation and mass determination on an Agilent 6500 Series LC/Q-TOF MS. The innovator and biosimilar were compared for sequence similarity, oxidation and deamidation status (Figure 1).
To probe the differences between the peptide maps of innovator and biosimilar, a mirror plot of TIC was generated using the Compare Files feature of Agilent MassHunter Comparative Analysis software (Figure 2).
Integrated Agilent systems deliver fast, accurate, high-resolution peptide mapping
- Agilent 1290 Infinity LC systems offer seamless transfer of methods between LCs, delivering unchanged retention time and peak resolution.
- An infinite power range that combines ultra high pressure up to 1200 bar and high flow rates up to 5 mL/min for maximum chromatographic performance, compatibility, flexibility, and investment protection.
- The revolutionary Agilent Max-Light cartridge cell with 60 mm optical path length provides ultra sensitivity.
- Agilent 6550 iFunnel Q-TOF LC/MS delivers the full power of high-resolution accurate mass MS/MS detail and configurable for research or routine applications.
- Ion transfer is increased to achieve lowest detection levels of any high resolution LC/MS.
- Realize 5x greater on-column sensitivity and increases up time to maintain the highest sensitivity for high throughput applications.
- Agilent MassHunter BioConfirm software and Agilent MassHunter Comparative Analysis software provide automated data extraction, sequence matching, PTM identification, and sequence coverage calculation.
Real world example: Agilent solution used in peptide mapping of innovator pharmaceutical and biosimilar
In our example, Rituximab biosimilar and innovator were purchased from a pharmacy and stored according to the manufacturer’s instructions. Additionally, DL-Dithiothreitol (DTT), idoacetamide, Trisbase, high-quality sequence grade trypsin, and LC/MS grade solvents were purchased.
Since peptide mapping is regarded as the "fingerprint" of the protein under analysis, it was an excellent technique for comparing the similarity between Rituximab and its biosimilar version. Peptide masses were obtained from the LC/MS run using the Molecular Feature Extraction feature of BioConfirm. Peptide maps were also used to quantify the extent of oxidation and deamidation as these are the two most commonly occurring modifications seen during storage, formulation, and sample handling.
Full details of this application can be found in Agilent publication 5991-6522EN.
Agilent has a full range of LC, LC/MS, and Q-TOF solutions for busy researchers and lab managers
Agilent has a wide array of Liquid Chromatography, LC/MS, and Q-TOF equipment, supplies, and related software that can increase throughputs in your busy research lab. Contact an Agilent representative today to learn more about these solutions and how they can improve the efficiency and cost-effectiveness of your laboratories.
Follow our Agilent Biopharma LinkedIn page for updates on Agilent biopharma applications, news, events and other information.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
All articles in this issue
 New Agilent 8900 ICP-QQQ delivers increased sensitivity, improved accuracy, and higher productivity
New Agilent 8900 ICP-QQQ delivers increased sensitivity, improved accuracy, and higher productivity New Agilent 1260 Infinity II LC system delivers higher level of operational efficiency
New Agilent 1260 Infinity II LC system delivers higher level of operational efficiency Robust Agilent 6470 Triple Quadrupole LC/MS delivers confident quantification and streamlined workflows
Robust Agilent 6470 Triple Quadrupole LC/MS delivers confident quantification and streamlined workflows Achieve accurate analysis of sulfur and nitrogen with the new and simplified Agilent 8355 SCD
Achieve accurate analysis of sulfur and nitrogen with the new and simplified Agilent 8355 SCD Agilent 1290 LC and 6550 iFunnel Q-TOF combine to provide fast, high-resolution peptide mapping of innovator and biosimilar mAbs
Agilent 1290 LC and 6550 iFunnel Q-TOF combine to provide fast, high-resolution peptide mapping of innovator and biosimilar mAbs Agilent collaborates with Academia—from investigating arsenic mysteries to understanding the complexity of biology
Agilent collaborates with Academia—from investigating arsenic mysteries to understanding the complexity of biology Excellent inertness for analysis of challenging polar compounds
Excellent inertness for analysis of challenging polar compounds Detection of semivolatile extractables and leachables (E&Ls) found in pharmaceutical products
Detection of semivolatile extractables and leachables (E&Ls) found in pharmaceutical products Agilent multi-omics solutions unravel the effects of low-level gamma radiation on rice seeds
Agilent multi-omics solutions unravel the effects of low-level gamma radiation on rice seeds Agilent comprehensive water screening solution reliably identifies wastewater contaminants of emerging concern:
Agilent comprehensive water screening solution reliably identifies wastewater contaminants of emerging concern:
A real-life application
Figure 1

Total ion chromatogram (TIC) of trypsin-digested mAb obtained using LC/MS for (A) innovator and (B) biosimilar with an Agilent AdvanceBio peptide mapping column.
Figure 2

Agilent MassHunter BioConfirm Software Comparative Analysis feature. (A) Mirror plot of TIC between innovator (red trace) and biosimilar (blue trace). The region at around 14.4 minutes is highlighted to show the difference of SLSLSPGK peptide. (B) The EIC of SLSLSPGK peptide shows that it is enriched in biosimilar. Similarly, the peak around 18.7 minutes corresponds to SLSLSPG peptide (lysine truncated). (C) The EIC of SLSLSPG peptide shows it is enriched in innovator.