PD-L1 IHC 22C3 pharmDx Overview

- With indications across a wide range of cancer types, oncologists are increasingly selecting patients for treatment with KEYTRUDA
- PD-L1 IHC 22C3 pharmDx is the clinically relevant option when considering patients for treatment with KEYTRUDA
High specificity for accurate clinical results
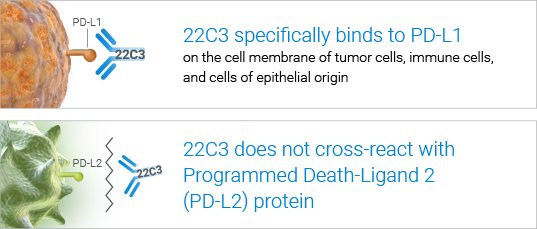
High sensitivity for precise PD-L1 detection
- Assessment of PD-L1 expression in NSCLC demonstrated staining across the dynamic range of TPS 0–100% and 0–3+ staining intensities
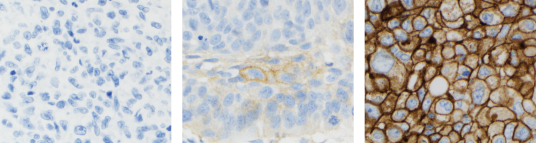
The stains above are of NSCLC specimens stained with PD-L1 IHC 22C3 pharmDx (SK006).

- Agilent performed rigorous repeatability testing to ensure reliable results
- 100% overall agreement across inter-instrument, inter-day, and intra-run tests†

- Reproducibility testing performed at three external testing sites demonstrated > 85% overall agreement across all tests†
- Inter-site, intra-site, inter-observer, and intra-observer tests were all performed

- PD-L1 IHC 22C3 pharmDx comes pre-validated, including validated scoring guidelines and on‑site product demonstrations
- All necessary components for a complete staining run are validated together and included in each kit
†Overall agreement is based on average negative and average positive agreements. Tests were performed using NSCLC tissue. Refer to the local Instructions for Use for overall agreement data for other commercially available indications.
- PD-L1 IHC 22C3 pharmDx is a standardized IHC assay with all necessary components for 50 tests in one kit1
- Designed for use on Autostainer Link 48, which comes preprogrammed with a validated staining protocol in the DakoLink software1

Intended Use
For in vitro diagnostic use.
PD-L1 IHC 22C3 pharmDx is a qualitative immunohistochemical assay using monoclonal mouse anti-PD-L1, Clone 22C3, intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC), urothelial carcinoma, esophageal cancer, head and neck squamous cell carcinoma (HNSCC), triple-negative breast cancer (TNBC), cervical cancer, and melanoma tissues using EnVision FLEX visualization system on Autostainer Link 48.
PD-L1 protein expression in NSCLC is determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity.
PD-L1 protein expression in urothelial carcinoma, esophageal cancer, TNBC, and cervical cancer is determined by using Combined Positive Score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100.
PD-L1 protein expression in HNSCC is determined by using CPS and/or TPS.
PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying patients for treatment with the therapies for the indications listed in Table 1.
Table 1: PD-L1 IHC 22C3 pharmDx companion diagnostic indications, PD-L1 expression levels, and therapies
| Tumor Indication | PD-L1 Expression Level | Therapy |
| NSCLC | TPS ≥ 1% TPS ≥ 50% |
|
| Urothelial Carcinoma | CPS ≥ 10 | |
| Esophageal Cancer | CPS ≥ 10 | KEYTRUDA®* |
| HNSCC | CPS ≥ 1 TPS ≥ 50% |
|
| TNBC | CPS ≥ 10 | |
| Cervical Cancer | CPS ≥ 1 | |
| NSCLC | TPS ≥ 50% | LIBTAYO®** |
* See the KEYTRUDA® product label for PD-L1 expression cutoff values and specific clinical circumstances guiding therapy.
** See the LIBTAYO® product label for specific clinical circumstances guiding therapy.
KEYTRUDA is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA
LIBTAYO is a registered trademark of Regeneron Pharmaceuticals, Inc.
References: 1. PD-L1 IHC 22C3 pharmDx [Instructions for Use]. Santa Clara, CA: Agilent Technologies, Inc.; 2022. 2. Keytruda [Summary of Product Characteristics]. European Medicines Agency; 2022. 3. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540–1550. 4. Reck M, Rodríguez-abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. 5. Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392–397.
For countries outside of the European Union, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.
For countries outside of the European Union, see the local LIBTAYO product label for approved indications.