Access Agilent eNewsletter May 2016

Agilent AdvanceBio SEC delivers optimal performance regardless of instrument
Linda Lloyd, Agilent Biocolumns Product Manager
Agilent’s new size exclusion biocolumn the AdvanceBio SEC has been developed for fast and accurate size-based characterization of therapeutic proteins, such monoclonal antibodies (mAb). The presence of aggregates or degradants can have a significant impact upon the safety, and can result in immune responses [1]. Therefore quality control regulations for therapeutic proteins, such as ICH(Q6B), clearly state that aggregates must be resolved from the desired product and quantitated. During the development of new therapeutic proteins, the probability and amount of aggregation occurring must be determined for all stages of production. To support these development activities the analysis may need to be performed in different laboratories using different LC systems. So methods must deliver accurate reproducible data across a range of LC instruments and laboratories. Size exclusion chromatography (SEC) separates solutes depending on their size in solution and is the method of choice for the quantitation of protein aggregates.
Groundbreaking innovation from Agilent
The new Agilent AdvanceBio SEC biocolumn is packed with unique 2.7 µm, low binding, polymer coated silica particles. The unique manufacturing method controls pore size, structure and volume. This technology provides minimal nonspecific interactions ensuring that protein peaks are sharp and resolved. As detailed in the video and previous article the AdvancedBio SEC biocolumn overcomes many roadblocks traditionally associated with SEC, ending uncertainty, and allowing you to resolve protein aggregates and degradants with speed and confidence.
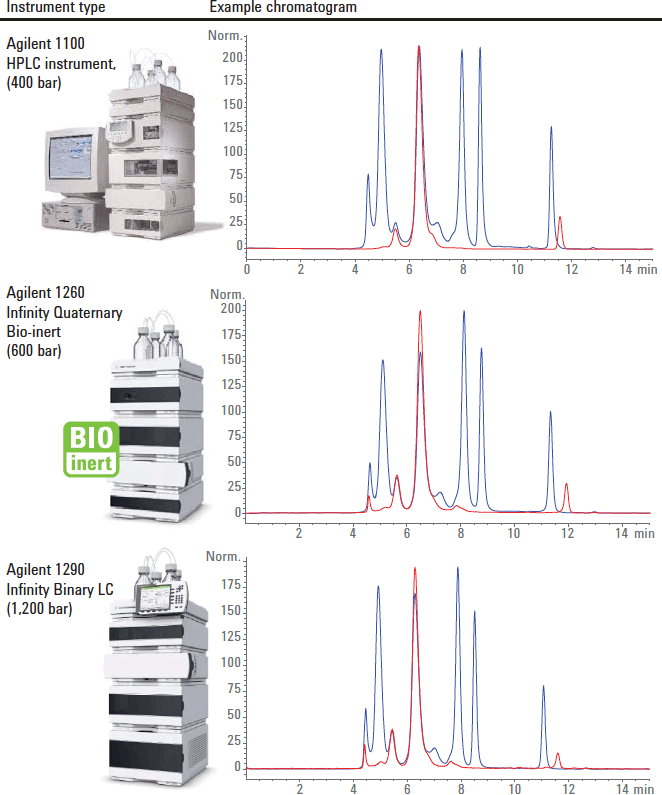
Figure 1. Illustrative examples of protein separations using different HPLC instruments, the blue trace is a BioRad protein standard mixture and the red is γ-globulin. These were different batches or proteins used in different laboratories.
Advances in Instrument compatibility with AdvancedBio SEC
While, recommended for use on an Agilent 1260 Infinity Bio-Inert LC system, Agilent AdvanceBio SEC columns have been developed to provide optimum performance regardless of the instrument used. The highly uniform 2.7 µm particles provide excellent column efficiency providing maximum protein recovery with operating pressures that allow operation on any HPLC system. Including 400 bar legacy instruments through to 1,200 bar UHPLC instrumentation. This extensive compatibility is illustrated in the chromatograms in Figure 1, which were generated in different laboratories, using different columns and different batches of protein. This compatibility with the full range of LCs means that there is no requirement to wait until a UHPLC system is available to generate the data, any available LC can be used.
Ensuring consistent results from instrument to instrument and laboratory to laboratory is essential for effective method transfer. The Agilent technical overview 5991-6302EN highlights the areas requiring consideration to obtain comparable SEC results on older 400 bar instruments through to modern UHPLC capable instrumentation, regardless of the location or operator. These include choosing the right column diameter. The Agilent AdvanceBio SEC biocolumn is available in two internal diameters, 4.6 and 7.8 mm. The use of columns of different internal diameters requires adjustments in flow rate, to maintain consistent linear velocity and sample volume. The ability to use smaller injection volumes for 4.6 mm columns is ideal when sample availability is limited for example. Whereas the larger sample volume of the 7.8 mm column is advantageous where robustness is required and light scattering detectors are used to characterize the aggregates.
Temperature is a factor often overlooked, as most separations are performed at ambient temperature. However temperature can vary depending on time of day, season or by heating or air conditioning. With aqueous separations, backpressure will vary noticeably with temperature in addition the level of aggregation may be effected. To ensure the effective transfer of methods, separations should be performed with the column contained in a thermostatically controlled oven so the samples are maintained in a temperature-controlled environment.
With an isocratic separation mechanism the most likely explanations for differences in retention time between instruments are either: Inaccuracies in the flow rate for example due to pump wear and tear. Or differences in the system dispersion volumes, extra column dead volume, changes in capillary length or diameter, or sample loop size. To determine whether factors other than the column should be optimized a protein standard mixture such as the Agilent AdvanceBio SEC protein standards, can be run and the peak shapes and efficiencies calculated and compared to the expected values.
Full details on this work are freely available in the technical overview 5991-6302EN. See our technical note 5991-6474EN about the benefits of Agilent AdvanceBio SEC columns for biopharmaceutical analysis to find out more, alternatively, read our brochure 5991-6212EN or application brief 5991-6424EN.
Agilent AdvanceBio SEC columns offer a breakthrough technology that has been developed to provide optimum performance regardless of the instrument, location or operator. This reproducibility allows you to resolve protein aggregates with speed and confidence, ending uncertainty. Agilent AdvanceBio SEC columns deliver reproducible results—column to column, batch to batch, and lab to lab.
Agilent offers a wide range of innovative biopharma solutions
The AdvanceBio SEC is the most recent member of Agilent’s innovative AdvanceBio family of HPLC columns. These columns are easy to integrate into your workflows and are available in a wide range of pore sizes and dimensions. Agilent delivers consistent, exceptional performance for the complete characterization of proteins, antibodies, conjugates, new biological entities and biopharmaceuticals. Explore our family of biologics solutions and LC biocolumns, then contact your Agilent Representative and see how our solutions can speed up your workflows and reduce the time to market for your new biotherapeutics.
Reference
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry
Immunogenicity Assessment for Therapeutic Protein Products. August 2014
For Research Use Only. Not for use in diagnostic procedures.
This information is subject to change without notice.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
All articles in this issue
 Detailed analysis of therapeutic proteins with Agilent 1290 Infinity II 2D-LC Solution
Detailed analysis of therapeutic proteins with Agilent 1290 Infinity II 2D-LC Solution Agilent AdvanceBio SEC delivers optimal performance regardless of instrument
Agilent AdvanceBio SEC delivers optimal performance regardless of instrument Quick, accurate pesticide analysis in foods using Agilent J&W DB-35ms Ultra Inert GC columns
Quick, accurate pesticide analysis in foods using Agilent J&W DB-35ms Ultra Inert GC columns Agilent Mini-QuEChERS and HES provide robustness and cost-savings in GC/MS/MS pesticide analysis of foods
Agilent Mini-QuEChERS and HES provide robustness and cost-savings in GC/MS/MS pesticide analysis of foods Agilent OpenLAB Electronic Lab Notebook gives biopharma company a competitive edge
Agilent OpenLAB Electronic Lab Notebook gives biopharma company a competitive edge Improved efficiency in routine analysis of hydride and non-hydride elements using Agilent MSIS
Improved efficiency in routine analysis of hydride and non-hydride elements using Agilent MSIS Maximize lab productivity and results consistency with Agilent SQ Walkup Solution
Maximize lab productivity and results consistency with Agilent SQ Walkup Solution Agilent Automated Purification Solutions help researchers to purify complex compounds
Agilent Automated Purification Solutions help researchers to purify complex compounds
Figure 1

Illustrative examples of protein separations using different HPLC instruments, the blue trace is a BioRad protein standard mixture and the red is γ-globulin. These were different batches or proteins used in different laboratories.