Access Agilent eNewsletter May 2016

Detailed analysis of therapeutic proteins with Agilent 1290 Infinity II 2D-LC Solution
Gurmil Gendeh, Agilent Director of Biopharma Segment Marketing
Protein biopharmaceuticals such as monoclonal antibodies and recombinant proteins are in widespread use for the treatment of various life-threatening diseases that include cancer and autoimmune diseases. Protein therapeutics have a complexity that far exceeds that of small-molecule drugs, and unraveling this complexity represents an analytical challenge. In this article, we present two examples to illustrate how two-dimensional liquid chromatography (2D-LC) using the Agilent 1290 Infinity II 2D-LC Solution delivers the resolving power you need for these difficult separations.
In-depth characterization of monoclonal antibody tryptic digests
Detailed characterization is a must during the development and lifetime of therapeutic macromolecules. Our first example describes a peptide mapping method for identity and purity assessment of the monoclonal antibody trastuzumab. This monoclonal antibody (mAb), marketed as Herceptin, is a 150 kDa molecule used in the treatment of breast cancer. Tryptic digestion gives more than 100 peptides that vary in physicochemical properties and are present in concentrations that span a wide dynamic range. This complex analytical challenge requires the highest separation power.
Comprehensive 2D-LC (LCxLC) substantially increases the chromatographic resolution if the two dimensions are orthogonal and the separation obtained in the first dimension is maintained upon transfer to the second dimension. The combination of strong cation exchange (SCX) and reversed-phase liquid chromatography (RPLC) provides good orthogonality towards the analysis of peptides. An Agilent 1290 Infinity II 2D-LC Solution proved highest performance and reliability for this analysis.
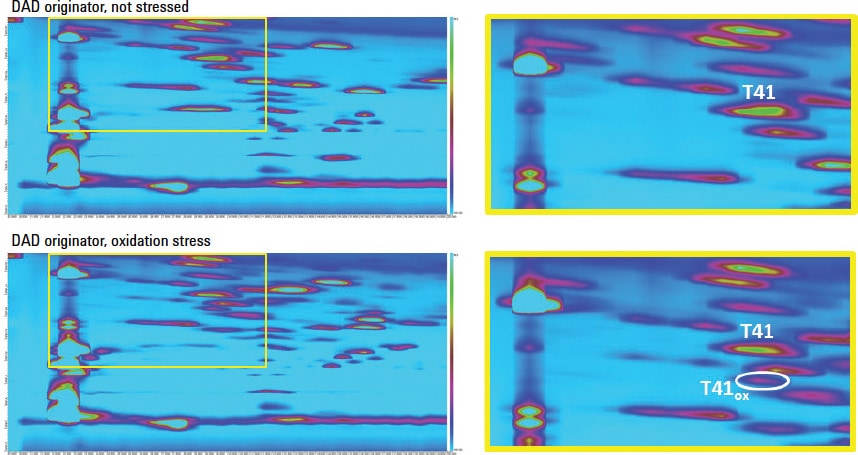
Figure 1. LCxLC contour plot for the analysis of a tryptic digest of non-stressed and oxidatively stressed trastuzumab shows location of modifications.
Separate impurities and modifications in very complex samples
In this highly stable analysis, 2D-LC was used to detect impurities and modifications of trastuzumab. A forced degradation study was performed, where a trastuzumab sample was stressed under both oxidative conditions and elevated pH conditions, which resulted in oxidation and deamidation, respectively. Figure 1 shows diode-array detector (DAD) results from an originator sample and an oxidation stressed sample. The stressed sample clearly contains additional spots. One spot was chosen for LC/MS/MS identification. The spot was traced back to peptide T41, and is the result of an oxidation of methionine present in this fragment.
This method demonstrated great potential for purity and identity assessment. The precision of the set-up was excellent, which allows use of the method for quality control and comparative studies between production batches and originators and biosimilars. Full details are available in Agilent Application Notes 5991-2880EN and 5991-4530EN.
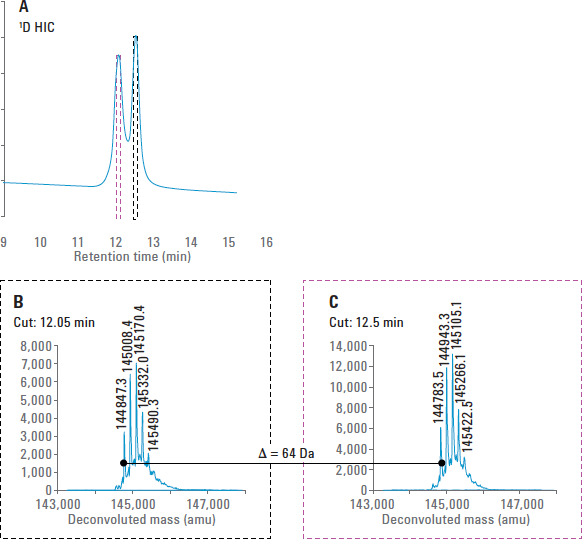
Figure 2. Analysis of mAb B with the HIC/RP 2D-LC/MS system. The mass of the oxidized mAb was 64 Da higher than that of the untreated mAb, which indicated four potential sites of oxidation.
Detect with MS, even if your separation is incompatible
In a second example, multiple heart-cutting (MHC) 2D-LC/MS was used combining hydrophobic interaction and reversed phase chromatography to analyze therapeutic proteins. Hydrophobic interaction chromatography (HIC) is a popular approach for both downstream processing and analytical-scale analysis of mAbs. These investigations often benefit from mass measurement using MS, but the high-salt conditions used for HIC separations are completely incompatible with electrospray ionization (ESI). This obstacle was overcome with the Agilent 1290 Infinity II 2D-LC Solution, which affords multiple heart-cutting and subsequent desalting/separation using reversed-phase (RP) chromatography on-line with TOF MS. For these experiments, described in Agilent Application Note 5991-6376EN, an Agilent 6224 Accurate-Mass TOF LC/MS system with a dual-nebulizer ESI source was used.
Accurate mass measurements of overlapped peaks
Additionally, mAb B was subjected to forced oxidation, which produced a peak that eluted 0.5 minutes earlier than the main mAb peak at 12.5 minutes (Figure 2A). Multiple heart-cutting was used to select each of the two peaks for mass measurement by 2D-LC/MS. As expected, the peak that resulted from oxidation was higher in mass than the main mAb peak (Figures 2B and 2C). In this case, the mass was 64 Da higher, which is consistent with four oxidation reactions. Based on prior knowledge of the molecule, these oxidation events likely occurred at the four methionine residues present in the mAb.
Remove limitations on separation time
MHC allows you to cut and store regions of interest from the first dimension, so you can allocate whatever time is necessary to conduct optimized separations in the second dimension. In the current work, adequate time could be allotted for sample desalting and sensitive introduction into the MS at a relatively low flow rate (0.2 mL/min). Even when desalting for MS analysis is not required, MHC-style 2D-LC allows you to perform longer 2D gradients, which are often needed with protein samples.
Optimize difficult LC separations
The Agilent 1290 Infinity II 2D-LC Solution delivers the ultimate resolution you need for the analysis of complex samples such as protein biopharmaceuticals. To discover more, watch a video about easy-to-use 2D-LC and read an Analytical Chemistry article about 2D-LC/MS of the therapeutic mAb rituximab. Then contact your Agilent representative for further information about 2D-LC.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
All articles in this issue
 Detailed analysis of therapeutic proteins with Agilent 1290 Infinity II 2D-LC Solution
Detailed analysis of therapeutic proteins with Agilent 1290 Infinity II 2D-LC Solution Agilent AdvanceBio SEC delivers optimal performance regardless of instrument
Agilent AdvanceBio SEC delivers optimal performance regardless of instrument Quick, accurate pesticide analysis in foods using Agilent J&W DB-35ms Ultra Inert GC columns
Quick, accurate pesticide analysis in foods using Agilent J&W DB-35ms Ultra Inert GC columns Agilent Mini-QuEChERS and HES provide robustness and cost-savings in GC/MS/MS pesticide analysis of foods
Agilent Mini-QuEChERS and HES provide robustness and cost-savings in GC/MS/MS pesticide analysis of foods Agilent OpenLAB Electronic Lab Notebook gives biopharma company a competitive edge
Agilent OpenLAB Electronic Lab Notebook gives biopharma company a competitive edge Improved efficiency in routine analysis of hydride and non-hydride elements using Agilent MSIS
Improved efficiency in routine analysis of hydride and non-hydride elements using Agilent MSIS Maximize lab productivity and results consistency with Agilent SQ Walkup Solution
Maximize lab productivity and results consistency with Agilent SQ Walkup Solution Agilent Automated Purification Solutions help researchers to purify complex compounds
Agilent Automated Purification Solutions help researchers to purify complex compounds
Figure 1

LCxLC contour plot for the analysis of a tryptic digest of non-stressed and oxidatively stressed trastuzumab shows location of modifications.
Figure 2

Analysis of mAb B with the HIC/RP 2D-LC/MS system. The mass of the oxidized mAb was 64 Da higher than that of the untreated mAb, which indicated four potential sites of oxidation.