Access Agilent eNewsletter July 2016

Analysis of PEGylated proteins with Agilent AdvanceBio SEC columns
M. Sundaram Palaniswamy, Agilent Senior Application Scientist
PEGylation is the process of covalent attachment of polyethylene glycol polymer chains to another molecule, normally a drug or therapeutic protein. PEGylation is routinely achieved by incubation of a reactive derivative of PEG with the target macromolecule. The PEG moiety offers numerous advantages for increasing a protein’s stability and circulating half-life. PEG has been approved by the Food and Drug Administration (FDA) as “generally recognized as safe” [1]. There are over 10 different PEGylated products currently approved by the FDA, with many more potential products in development. PEG GCSF is a long-acting form of recombinant GCSF. The SEC HPLC technique is recommended for determining the purity and higher aggregates [2].
Non-ideal adsorption of therapeutic proteins can disrupt SEC
Most published methods for PEG GCSF use aqueous mobile phases containing 100 mM NaCl, 85% orthophosphoric acid, and up to 10% ethanol to prevent nonspecific interactions and improve peak shape and resolution [3]. non-ideal adsorption of therapeutic proteins is a matter of concern during SEC, as aggregates sometimes show a greater tendency to bind to the stationary phase than the native form. Due to this preferential binding, SEC analysis of aggregates is inaccurate and there is the danger that aggregates may not be detected. Mobile phases containing organic solvents or extremes of pH have been used to overcome this problem and have been shown to enhance resolution and recovery. But as well as possibly dissociating reversible aggregates, they may also dissociate aggregates that are irreversible in the formulation buffer [4].
Agilent AdvanceBio SEC and 1260 LC deliver superior detection and separation
The Agilent AdvanceBio SEC, 130 Å, 7.8 x 300 mm, 2.7 µm column, a breakthrough technology for SEC analysis, contains an innovative silica particle and unique hydrophilic bonding chemistry. This column delivers resolution and size separations over a wide range of sample types, often without the need to add organic modifiers to the mobile phase. To demonstrate this capability, a completely biocompatible Agilent 1260 Infinity Bio-inert Quaternary LC System with a maximum pressure of 600 bar consisting of the following modules was used:
- Agilent 1260 Infinity Bio-inert Quaternary LC Pump
- Agilent 1260 Infinity Bio-inert High Performance Autosampler
- Agilent 1200 Infinity Series Thermostat
- Agilent 1260 Infinity Thermostatted Column Compartment containing Bio-inert click-in heating elements
- Agilent 1260 Infinity DAD VL (with Bio-inert standard flow cell)
- Agilent AdvanceBio SEC, 130 Å, 7.8 x 300 mm packed with 2.7 µm particles
The chromatographic parameters used in this example are described in Table 1.
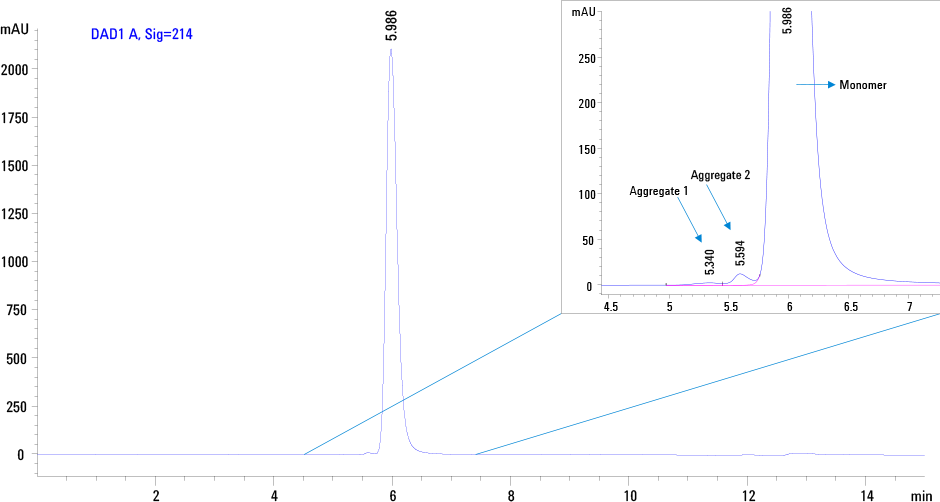
Figure 1. SEC profile of intact therapeutic PEG GCSF on an Agilent AdvanceBio SEC, 130 Å, 7.8 x 300 mm, 2.7 µm column.
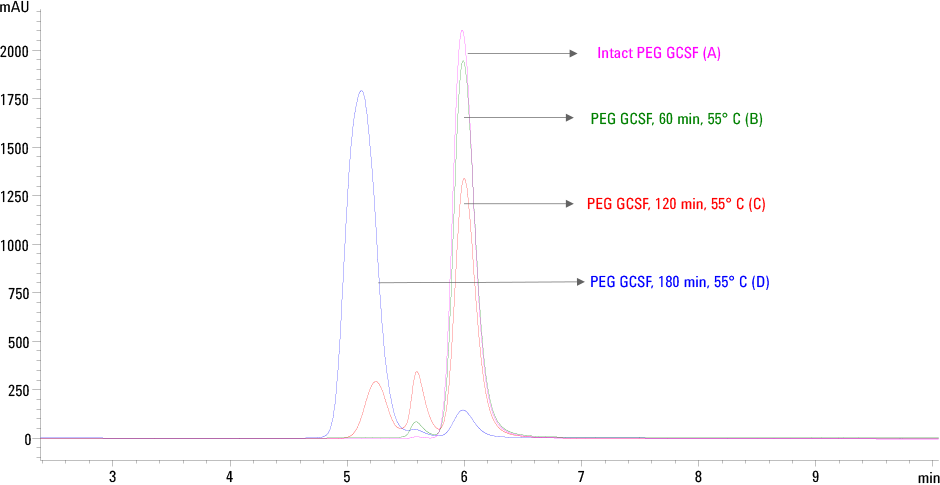
Figure 2. Trend of PEG GCSF aggregation determined by SEC HPLC on Agilent AdvanceBio SEC column. (A) Intact PEG GCSF, control (B) 60 min at 55 °C (C) 120 min at 55 °C and (D) 180 min at 55 °C.
| Parameters | Conditions |
|---|---|
| Mobile phase | 150 mM Sodium phosphate buffer, pH 6.8 |
| TCC temperature | Ambient |
| Isocratic run | Mobile phase A |
| Injection volume | 10 µL |
| Flow rate | 0.8 mL/min |
| UV detection | 214 and 280 nm |
Table 1. Chromatographic parameters used for SEC HPLC.
Results and Discussion
Figure 1 demonstrates the excellent separation of intact PEG GCSF as single symmetrical peak at 5.989 min under the chromatographic conditions. From this example, it is evident that the conjugate contains dimers and higher aggregates as indicated by arrows in the enlarged view. However the sample does not contain free GCSF as indicated by the absence of a late eluting peak.
The SEC profiles of heat stressed PEG GCSF shown in Figure 2 indicates that the Agilent AdvanceBio SEC column was able to separate and detect aggregates. Intact and higher aggregates of PEG GCSF are distinctly separated from each other as seen in the chromatogram. Based on the area percent, the relative quantitation of monomer and aggregates of PEG GCSF are summarized in Table 2.
| Stressed PEG GCSF (60 min) | Stressed PEG GCSF (120 min) | Stressed PEG GCSF (180 min) | |||
|---|---|---|---|---|---|
| Time | Area % | Time | Area % | Time | Area % |
| 5.59 | 2.85 | 5.24 | 16.01 | 5.12 | 91.89 |
| 5.99 (monomer) | 96 | 5.59 | 12.74 | 5.57 | 1.14 |
| 5.99 (monomer) | 70.29 | 5.98 (monomer) | 6.44 | ||
Table 2. Relative quantification of monomer and aggregates based on peak area.
It is evident from the data that there was an increase in the levels of aggregates when stressed at 55 °C. With a relative decrease in the amount of the monomeric form from 96 % to 70 and 6.44 % respectively.
Agilent offers a wide range of solutions at every stage in your process
Here, we showcase an excellent solution for analysis of PEGylated proteins using PEG GCSF as a model protein. A simple method for SEC HPLC using the Agilent AdvanceBio SEC column was developed for monitoring the purity of PEG GCSF without the use of organic modifiers in the mobile phase. Stress studies of therapeutic PEG protein demonstrated that the AdvanceBio SEC column was able to separate, detect and quantify aggregates based on area percent. Such a simple and reproducible method, coupled with the bio-inertness and corrosion resistance of the instrument, makes this solution reliable and suitable for the routine QC of PEGylated proteins for the Biopharma industry.
For a more detailed version of this application with additional data, please refer to the Agilent Application Note 5991-6791EN. Then explore our literature, videos, and e-Seminars to learn how Agilent solutions can increase throughputs and efficiencies in your busy laboratory.
References:
- Gaberc-Porekar, V, Zore, I, Podobnik, B, Menart, V. “Obstacles and pitfalls in the PEGylation of therapeutic proteins.” Current Opinion in Drug Discovery and Development, 11, 242–250 (2008).
- ipc.nic.in/writereaddata/monoprepimages/Pefilgrastim-2961377726.pdf
- Ratto JJ, O’Conner SR, Distler AR, Wu GM, Hummel D, Treuheit MJ, Herman AC, Davis JM. “Ethanol-sodium chloride-phosphate mobile phase for size-exclusion chromatography of poly (ethylene glycol) modified proteins.” Journal of Chromatography A 763:337–344. (1997).
- Tsutomu Arakawa et al. “The Critical Role of Mobile Phase Composition in Size Exclusion Chromatography of Protein Pharmaceuticals.” Journal of Pharmaceutical Sciences, Vol. 99, 1674–1692 (2010).
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
All articles in this issue
 Make your Q-TOF LC/MS analyses faster, easier, and more productive—regardless of your application
Make your Q-TOF LC/MS analyses faster, easier, and more productive—regardless of your application Agilent J&W DB-624UI capillary GC column excels for challenging applications with active compounds
Agilent J&W DB-624UI capillary GC column excels for challenging applications with active compounds Tip: Using enzymes in dissolution testing of gelatin capsules
Tip: Using enzymes in dissolution testing of gelatin capsules InfinityLab Poroshell 120 columns maximize LC workflow efficiency
InfinityLab Poroshell 120 columns maximize LC workflow efficiency Emerging life science applications of FTIR Imaging: Providing spatially resolved molecular information in disease research
Emerging life science applications of FTIR Imaging: Providing spatially resolved molecular information in disease research New LC/TQ database and method for central carbon metabolites
New LC/TQ database and method for central carbon metabolites Agilent QQQ systems enable ground-breaking research on use of metabolite biomarkers to predict a common pregnancy complication
Agilent QQQ systems enable ground-breaking research on use of metabolite biomarkers to predict a common pregnancy complication Analysis of PEGylated proteins with Agilent AdvanceBio SEC columns
Analysis of PEGylated proteins with Agilent AdvanceBio SEC columns Structural elucidation of in-process impurities using high resolution LC/MS/MS
Structural elucidation of in-process impurities using high resolution LC/MS/MS Removal of lipids for the analysis of toxicological compounds in plasma by LC/MS/MS
Removal of lipids for the analysis of toxicological compounds in plasma by LC/MS/MS Quick, accurate, cost-effective measurements of veterinary drugs in meat with Agilent MS/MS, UHPLC, and Q-TOF
Quick, accurate, cost-effective measurements of veterinary drugs in meat with Agilent MS/MS, UHPLC, and Q-TOF
Figure 1

SEC profile of intact therapeutic PEG GCSF on an Agilent AdvanceBio SEC, 130 Å, 7.8 x 300 mm, 2.7 µm column.
Figure 2

Trend of PEG GCSF aggregation determined by SEC HPLC on Agilent AdvanceBio SEC column. (A) Intact PEG GCSF, control (B) 60 min at 55 °C (C) 120 min at 55 °C and (D) 180 min at 55 °C.