Access Agilent eNewsletter July 2016
Tip: Using enzymes in dissolution testing of gelatin capsules
G. Bryan Crist, Agilent Scientific Affairs Manager
A concern identified with the existing Dissolution Chapter has been the efficiency of enzymes for two-tier testing in the event of a dissolution failure due to cross-linking. A modification to USP Chapters: Dissolution and Disintegration and Dissolution of Dietary Supplements has been published in the US Pharmacopeial Forum. [1] In March of 2014, at USP headquarters in Rockville, MD, a workshop was conducted to address the ineffectiveness of presently used pepsin and pancreatin in handling dissolution of gelatin capsules exhibiting crosslinking in media with a mid-range pH of about 4.5 to 6.5 units. [2,3]
Cross-linking can render dissolution testing and monitoring useless
Cross-linking is defined as the “formation of strong chemical linkages beyond simple hydrogen and ionic bonding between gelatin chains.” The reaction itself is:
- Generally irreversible
- Renders the gelatin insoluble
- Catalyzed by a number of chemical and environmental factors [1]

Figure 1. Cross-linking and pellicle formation.
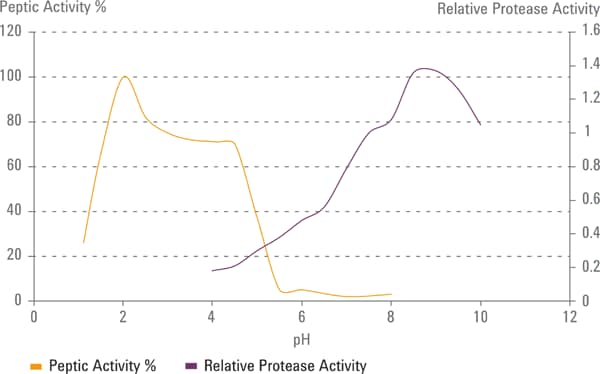
Figure 2. Peptic and protease activity with respect to pH.
The impact of cross-linking is a delay or inability of the capsule shell to open due to pellicle formation on the internal or external gelatin surface (Figure 1). This is visually confirmed with the presence of thin, rubbery-appearing membranes or gelatinous masses. Occasionally, thin wispy strands of gelatin may be observed, indicating early signs of cross-linking during stability studies.
The current ICH-harmonized USP requirement for hard or soft gelatin capsules and gelatin-coated tablets that do not conform to the dissolution specifications is:
Where water or medium with a pH of less than 6.8 is specified as the Medium in the individual monograph, the same Medium specified may be used with the addition of purified pepsin that results in an activity of 750,000 Units or less per 1000 mL – for media with a pH of 6.8 or greater, pancreatin can be added to produce not more than 1750 USP units of protease activity per 1000 mL.[2]
The problem with this approach is that the activity of pepsin peaks around pH 2 and has good activity up to about pH 4.5. Pancreatin peak activity is about pH 6.8 with good activity between 6-8 units. This creates a problem for products requiring media in the range of about 4 to 6.8 because the listed enzymes do not have sufficient activity in this range, as illustrated in Figure 2.
Two new enzymes shown promising in elimination of cross-linking
The USP workshop suggested two new enzymes with sufficient activity in mid-range pH media: papain and bromelain. [3] These were also included in the US Pharmacopeial Forum for inclusion into the USP Dissolution chapter. Papain is derived from unripe green papaya while bromelain is derived from pineapple stems. Both have an optimal pH around 5 and exhibit good activity through the pH range of 4.0 to 6.8. The proposed activity levels are NMT 550,000 units/L for papain and NMT 30 gelatin-digesting units (GDU)/L of dissolution media for bromelain. The activity determinations are found in the USP under the monograph section for papain and under the Reagent Specifications for bromelain. Additionally, the continued use with pepsin will be clarified for use with pH ≤ 4.0.
Enzyme interaction with surfactant containing dissolution media and product formulations during the dissolution method development stages should be evaluated with “healthy” gelatin capsules. If additional testing is to be performed with enzymes due to cross-linking, the establishment of the proper enzyme for Tier 2 testing will have been verified and documented. To learn more about this topic, visit the Dissolution Discussion Group (DDG) page and listen to the May 2015 DDG quarterly meeting replay.
Agilent provides a wide range of dissolution and automated sampling solutions
Agilent offers a huge array of dissolution and automated sampling solutions, apparatus, accessories, and software that can dramatically improve analytic results in your lab. Additionally, you will find a wealth of information on dissolution, cross-linking, and other analytical topics on our web site; including articles, application notes, and videos. Contact an Agilent representative to learn more.
References
- USP Stimuli to the Revision Process, Use of Enzymes in the Dissolution Testing of Gelatin Capsules and Gelatin-Coated Tablets—Revisions. USP-PF 40(6)
- US Pharmacopeia 38, NF 33, Physical Test Dissolution; US Pharmacopeial Convention 12610 Twinbrook Parkway, Rockville, MD 20852
- Use of Enzymes in Dissolution Testing, Thomas Langdon—American Laboratories, Inc., Presented at USP Workshop on Dissolution testing of Capsules, March 24-25, 2014, USP, Rockville, MD
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
All articles in this issue
 Make your Q-TOF LC/MS analyses faster, easier, and more productive—regardless of your application
Make your Q-TOF LC/MS analyses faster, easier, and more productive—regardless of your application Agilent J&W DB-624UI capillary GC column excels for challenging applications with active compounds
Agilent J&W DB-624UI capillary GC column excels for challenging applications with active compounds Tip: Using enzymes in dissolution testing of gelatin capsules
Tip: Using enzymes in dissolution testing of gelatin capsules InfinityLab Poroshell 120 columns maximize LC workflow efficiency
InfinityLab Poroshell 120 columns maximize LC workflow efficiency Emerging life science applications of FTIR Imaging: Providing spatially resolved molecular information in disease research
Emerging life science applications of FTIR Imaging: Providing spatially resolved molecular information in disease research New LC/TQ database and method for central carbon metabolites
New LC/TQ database and method for central carbon metabolites Agilent QQQ systems enable ground-breaking research on use of metabolite biomarkers to predict a common pregnancy complication
Agilent QQQ systems enable ground-breaking research on use of metabolite biomarkers to predict a common pregnancy complication Analysis of PEGylated proteins with Agilent AdvanceBio SEC columns
Analysis of PEGylated proteins with Agilent AdvanceBio SEC columns Structural elucidation of in-process impurities using high resolution LC/MS/MS
Structural elucidation of in-process impurities using high resolution LC/MS/MS Removal of lipids for the analysis of toxicological compounds in plasma by LC/MS/MS
Removal of lipids for the analysis of toxicological compounds in plasma by LC/MS/MS Quick, accurate, cost-effective measurements of veterinary drugs in meat with Agilent MS/MS, UHPLC, and Q-TOF
Quick, accurate, cost-effective measurements of veterinary drugs in meat with Agilent MS/MS, UHPLC, and Q-TOF
Figure 1

Cross-linking and pellicle formation.
Figure 2

Peptic and protease activity with respect to pH.