Access Agilent eNewsletter April 2016

Simplify achiral-chiral analysis of warfarin metabolites using Agilent 2D-LC/MS solutions
Smriti Khera
Agilent Pharmaceutical Segment Marketing Manager
Siji Joseph
Agilent Applications Scientist
The analysis of drug metabolites in biological matrices is very challenging because analysts must contend with serious matrix interferences, as well as a low sensitivity requirement and dynamic range demands. Coelution can be an additional problem because oxidative metabolites are often isobaric with subtle or no differences in MS fragmentation patterns, requiring chromatographic or other orthogonal techniques for their resolution.
Chiral drug metabolites present additional challenges because from drug metabolism and regulatory perspectives, you must obtain clear resolution of your chiral metabolite enantiomers and have reproducible methods in place to identify and quantify them. The Agilent 1290 Infinity II 2D-LC Solution enables the resolution you need for this difficult separation problem.
Simultaneous, stereospecific resolution of achiral and chiral drug metabolites
In collaboration with scientists at Biocon Bristol-Myers Squibb Research Center (BBRC) in Bangalore, India, we developed a method to analyze chiral drug metabolites. The method uses two-dimensional LC (2D-LC) coupled to a high-resolution Agilent 6530 Accurate-Mass Quadrupole Time-of-Flight (Q-TOF) LC/MS. 2D-LC is ideally suited to this application because you can achieve achiral resolution of drug-related analytes from the sample matrix in the first dimension, followed by chiral resolution in the second dimension. The Agilent 1290 Infinity II 2D-LC Solution makes the application of two-dimensional chromatography and method development very accessible and reproducible for the bioanalyst.
Warfarin studies show the advantages of 2D-LC
We utilized multiple heart-cutting 2D-LC to the analysis of metabolites of warfarin (Coumadin)[1], which is an anticoagulant that has been used for more than 50 years. It is administrated as an equal mixture of R- and S- enantiomers. Each warfarin enantiomer is differentially cleared through the formation of several active and inactive stereo-isomeric oxidative metabolites. Oxidative warfarin metabolites are isobaric and many of them have similar MS/MS fragmentation patterns. A better understanding of inter-individual warfarin metabolism is critical for understanding the dose-response relationships during warfarin therapy. Together with fellow scientists at the BBRC in India, we studied warfarin metabolism using Agilent 2D-LC/MS Q-TOF as a proof-of-concept for our proposed workflow.
For this difficult separation, we employed an achiral reversed-phase column in the first dimension to isolate the five achiral hydroxywarfarins from each other and the parent warfarin, as well as from the matrix interferences. Each hydroxywarfarin was then transferred to the second dimension for chiral analysis using the multiple heart-cut approach. You can find details of this method in the on-demand webinar, as well as in a previous Access Agilent article.[2]
Successfully identify and quantify stereoisomers—in a single run
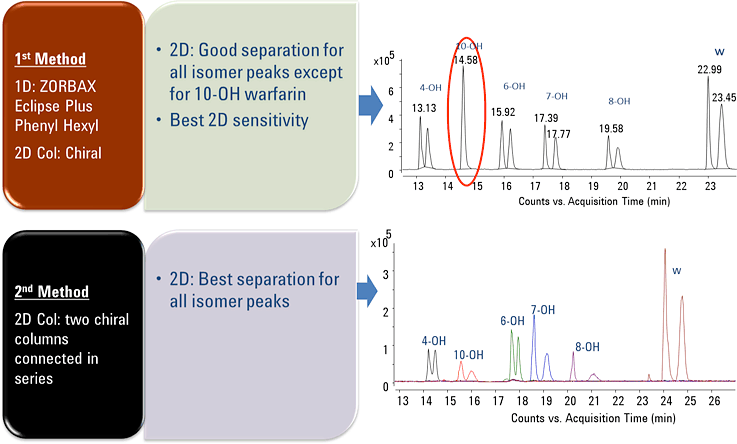
Figure 1. Successful enantiomer separation of warfarin and hydroxy metabolites using Agilent 2D-LC/Q-TOF method.
Figure 1 shows that using our method, we could simultaneously and stereospecifically resolve all twelve warfarin-related peaks with baseline separation. This separation allowed the accurate identification and quantification of each hydroxywarfarin stereoisomer. The method was linear across three orders of dynamic range for all analytes, with limits of detection (LODs) in the single-digit nM range. This approach was partially validated for robustness with excellent results.
We then used the method for the analyses of real-world samples derived from incubations of racemic and enantio-pure warfarin with human liver microsomes. We performed both a time course study as well as a 24-hour incubation of racemic warfarin with plated hepatocytes. The results from these studies were astonishing. From just a single chromatographic run at each time point, we could determine rates of formation for all metabolites and the rate of disappearance of parent drug (Figure 2). From these rates, we could calculate the intrinsic clearance (CLint) of each metabolite.
In addition, from just one analysis using our method, each hydroxywarfarin enantiomer could be accurately identified using a combination of retention time, high-resolution Q-TOF MS and MS/MS data (Figure 3) in a real metabolism sample.
Comprehensive solutions for comprehensive analyses
Our advanced Agilent 1290 Infinity II 2D-LC Solution coupled to a high resolution Q-TOF mass spectrometer provides a best-in-class solution for a very challenging but very common analytical problem faced by drug metabolism groups.
For more information on this application, watch this free webinar. Then explore new tips and tools that can help you bring pharmaceuticals to market faster.
Reference
- S. Joseph, M. Subramanian, and S. Khera. Simultaneous and stereospecific analysis of warfarin oxidative metabolism using 2D LC/Q-TOF, Bioanalysis 2015, 7(18), 2297-2309.
- S. Joseph, Multiple heart-cutting 2D-LC/ MS method for reproducible resolution of chiral drug metabolites, Access Agilent, March 2016.
Stay informed about the applications that are important to you
Subscribe to Access Agilent
Our free customized
monthly eNewsletter
Article Directory – April 2016
All articles in this issue
 Analyze low-ppm levels of active sulfur compounds with inert sample path on the Agilent 490 Micro GC
Analyze low-ppm levels of active sulfur compounds with inert sample path on the Agilent 490 Micro GC Fast arsenic speciation for food and urine analysis with Agilent LC-ICP-QQQ
Fast arsenic speciation for food and urine analysis with Agilent LC-ICP-QQQ Simplify achiral-chiral analysis of warfarin metabolites using Agilent 2D-LC/MS solutions
Simplify achiral-chiral analysis of warfarin metabolites using Agilent 2D-LC/MS solutions Optimized detection of gaseous sulfur compounds using Agilent J&W DB-Sulfur SCD GC column and Inert Flow Path
Optimized detection of gaseous sulfur compounds using Agilent J&W DB-Sulfur SCD GC column and Inert Flow Path Oil-free laboratory vacuum eliminates oil mess and disposal costs
Oil-free laboratory vacuum eliminates oil mess and disposal costs Eliminate conventional SEC roadblocks with Agilent AdvanceBio SEC columns
Eliminate conventional SEC roadblocks with Agilent AdvanceBio SEC columns Fast, economical assessment of herbals with Agilent 1290 Infinity LC system and LC columns
Fast, economical assessment of herbals with Agilent 1290 Infinity LC system and LC columns Agilent 5977B GC/MSD with HES offers improved VOC detection limits
Agilent 5977B GC/MSD with HES offers improved VOC detection limits
Figure 1

Successful enantiomer separation of warfarin and hydroxy metabolites using Agilent 2D-LC/Q-TOF method.
Figure 2
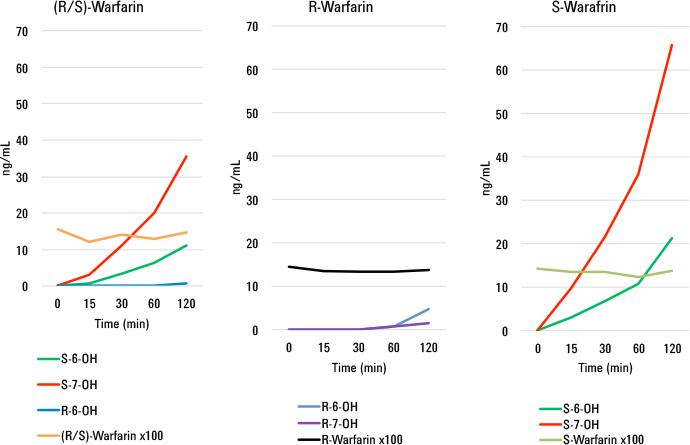
Rates of formation of hydroxywarfarins from racemic (R/S), (R )-, and (S)- warfarin time coursed microsomal incubations for a single chromatographic run.
Figure 3
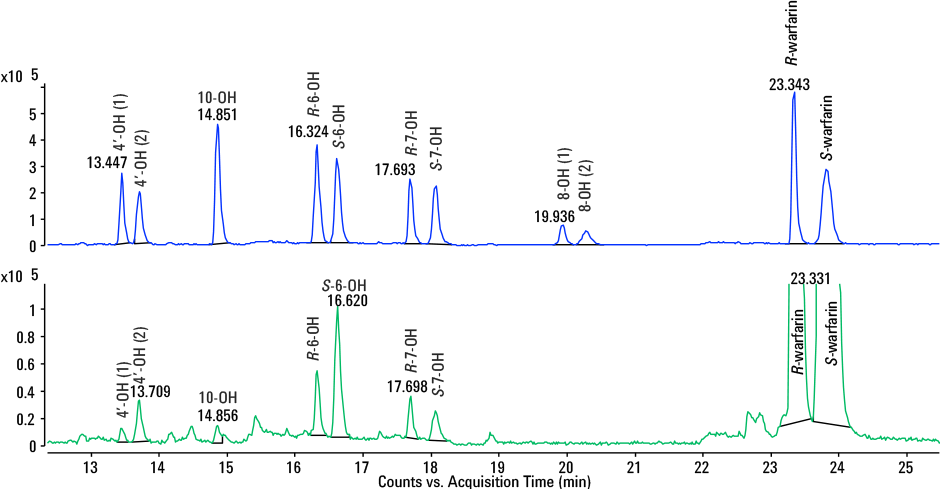
Extracted ion chromatogram of hepatocyte incubation at 24 hours (bottom) overlaid with standard mix (top). All hydroxylated metabolites were accurately identified and quantified.