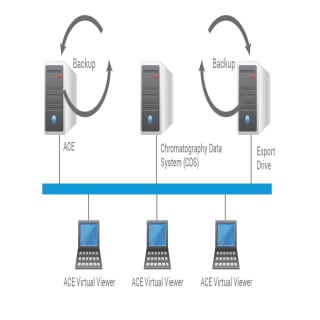
Network ACE
Agilent offers automated compliance engine (ACE), audit-ready, paperless software for instrument qualification and reporting. Specifically, we’ve enabled the installation of Network ACE within laboratory networks to simplify deployment and data integrity alignment. Like ACE, Network ACE is independent of your chromatography data systems (CDS), so you can harmonize USP <1058> analytical instrument qualification (AIQ) practices and simplify audit preparation. With ACE software, count on adherence to protocols, consistent calculations, and accurate reporting.
- Instrument & Software Qualification
Product Details
Features
- Network ACE: delivers added benefits that can help your lab improve efficiency, while reducing time and costs.
- Safely move your lab from Excel- or paper-based protocols toward coordinated AIQ processes for chromatography, spectroscopy, and other technologies
- Direct electronic data transfer from the chromatography data system (CDS)
- Secure, end-to-end traceability in qualification work
- Equipment qualification plans (EQPs) and reports (EQRs) are retained within your IT domain, ensuring data integrity and consistency.
- Designed to satisfy ALCOA+ data integrity requirements
- Software and data access are protected by the same IT policies, controls, security, backup, and archiving that apply to other systems within your validated IT environment
- Provides Enterprise-wide scalability
- Enhanced support of your cyber-security initiatives
How It Works
Literature
- Flyers
-
CrossLab NDA Compliance Flyer
Assured data integrity for analytical instrument qualification using Agilent CrossLab Network Distributed ACE (NDA)
- Flyers
- English
- 18 Oct 2024
- 441.25 KB
Digital Qualification Data and Report Management: Utilizing the Agilent EQR Manager
EQR Manager is installed on site as part of Network ACE, providing a secure centralized network location and easy to use tool to support electronic management and review of your Equipment Qualification.
- Flyers
- English
- 28 Feb 2024
- 315.77 KB
Scalable Qualification Solutions Agilent Network ACE Enhancement
This Network ACE update provides scalable ACE configurations — simplifying adding multiple sites for enhanced compliance and operational efficiency.
- Flyers
- English
- 02 Oct 2023
- 291.47 KB
Customer IT Responsibilities: Agilent Network ACE
Clarification of IT requirements, responsibilities, and explanation of Network ACE's function to simplify implementation.
- Flyers
- English
- 28 Feb 2023
- 524.18 KB
Agilent CrossLab Network Distributed ACE (Alcoa+)
Agilent CrossLab Network Distributed ACE: How it Satisfies ALCOA + Data Integrity Requirements
- Flyers
- English
- 29 Jul 2022
- 212.28 KB
- Technical Overviews
Videos
Related Products
Promotions