|
|
Our Focus: Improving Quality of Life
Genetic defects can be responsible for a wide range of disorders, from intellectual disability to congenital dysmorphisms, neuromuscular disorders, epilepsy and autism.
In addition, genetic defects can be responsible for Developmental Delay (DD) and Intellectual Disability (ID), including Autism Spectrum Disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD).
Many of these are copy-number variants, deleted or duplicated regions of the genome that can range in size from very small to entire chromosomes.
Although the average age of diagnosis for pervasive developmental disorder can be as late as four years old, research has shown that an early diagnosis can be critical2 to the prognosis of the child, as it can enable interventions that may prevent, anticipate or more successfully treat complications.3 Diagnosis can also facilitate financial support, educational assistance and membership in support groups.
|
The GenetiSure Dx Postnatal Assay
The GenetiSure Dx Postnatal Assay uses Agilent proprietary aCGH for copy-number and LOH data, enabling cytogeneticists to accurately detect genetic anomalies associated with developmental delay, intellectual disability, congenital anomalies, and dysmorphic features in children and adults.
|
Copy number changes are the causes of about 25% to 30% of all major congenital dysmorphisms1
Genetic anomalies account for 25% to 50% of ID cases, and this number increases with the severity of the disability
Median global prevalence of ASD alone was an estimated 62/10,000 in 2012
At least 10% of all neonatal intensive-care unit admissions involve the presence of congenital dysmorphisms2
|
|
|
|
What is aCGH and why?
Comparative Genomic Hybridization Arrays (aCGH) use a modified in situ hybridization technology, that allows detection and mapping of gDNA sequence copy differences between two genomes in a single experiment.
Analysis of fluorescence intensity of probes with respect to their genomic location enables detection of regions where copy-number variations (CNVs) and copy-neutral loss of heterozygosity (cnLOH) may occur.
|
 |
|
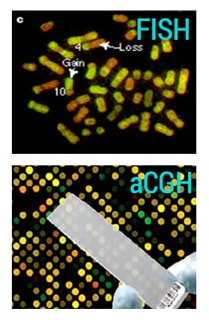
Figure: aCGH uses a two-color fluorophore hybridization that provides an easy visual identification and comparison of gains and deletions in the genome leading to copy number variation, between the test and reference samples. The results obtained with FISH offer less resolution and quantitative comparison between test and reference samples.
|
Higher diagnostic yield, increased resolution and greater sensitivity make aCGH a superior method compared with traditional karyotyping or FISH. Medical geneticists now consider aCGH to be the standard test for detecting CNVs linked to a unique patient or to one of the many known genetic disorders that cannot be defected by karyotyping alone.8,12,13
For more information, please visit www.agilent.com/chem/contactus.
|
|