PD-L1 IHC 22C3 pharmDx on Dako Omnis Overview

PD-L1 IHC 22C3 pharmDx (Dako Omnis) is now available for Dako Omnis:
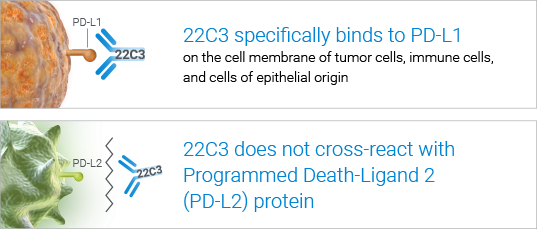
- PD-L1 IHC 22C3 pharmDx (Dako Omnis) uses the ONE 22C3 antibody proven throughout the KEYTRUDA Non-Small Cell Lung Cancer (NSCLC) clinical trials
- Identify NSCLC patients eligible for treatment with KEYTRUDA with a fully validated and CE-Marked assay designed for Dako Omnis1
- This combines the clinical relevance of PD-L1 22C3 pharmDx with the flexibility and choice of Dako Omnis to provide a fully automated PD-L1 diagnostic service integrated into the core of your laboratory workflow
Workflow optimized PD-L1 assay for more time, greater choice and better patient care
- Accelerate time to diagnosis with high throughput and continuous delivery of complete NSCLC cases to enable pathologists to report PD-L1 results in the context of your routine lung IHC panel
- Standardized IHC assay for 60 tests integrated into the Dako Omnis workflow to help you provide a quality PD-L1 service on the same instrument as your routine IHC
- Dako Omnis has a controlled on-board environment with fully optimized, standardized and validated protocols to ensure consistent results every time
- Developed, tested and validated to deliver results highly concordant with PD-L1 IHC 22C3 pharmDx on Autostainer Link 48 for diagnostic confidence
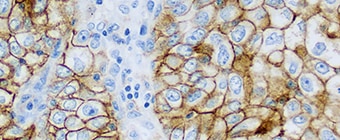
High specificity for accurate clinical results

Want to hear more about PD-L1 IHC 22C3 pharmDx?
Send us an email and we'll get back to you.
Send emailOr for more information, contact us.
Intended Use
PD-L1 IHC 22C3 pharmDx (Dako Omnis) is a qualitative immunohistochemical assay using Monoclonal Mouse Anti-PD-L1, Clone 22C3 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) Non-Small Cell Lung Cancer (NSCLC) tissue, using EnVision FLEX visualization system on Dako Omnis.
PD-L1 protein expression is determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity.
PD-L1 IHC 22C3 pharmDx (Dako Omnis) is indicated as an aid in identifying NSCLC patients for treatment with KEYTRUDA® (pembrolizumab) monotherapy. See the KEYTRUDA® product label for expression cutoff values guiding therapy in specific clinical circumstances guiding PD-L1 testing.
KEYTRUDA is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
References
1. PD-L1 IHC 22C3 pharmDx (Dako Omnis) [package insert]. Carpinteria, CA. Dako, Agilent Pathology Solutions, 2018.