The TRS100 quantitative pharmaceutical analysis system
TRS100 Quantitative Pharmaceutical Analysis System
A TRS100 system can reduce content uniformity, assay, and ID tests to minutes per batch, saving significant cost and speeding up your quality control workflow. Sample preparation and consumables are not needed, meaning skilled analytical resources are not required. Content uniformity testing methods using transmission Raman have been approved by regulators using ICH and regulatory authority-acceptable protocols.
- Raman Pharmaceutical Analysis Systems
Request a Quote
Product Details
- Assay hundreds of tablets or capsules in minutes.
- Works with intact capsules and coated tablets of most thicknesses.
- Requires no sample preparation, wet chemistry, or skilled testing resources.
- Quantifies APIs and excipients in a single measurement.
- Achieves a limit of quantification as low as 0.1% w/w.
- Quantifies polymorphs in intact tablets.
- Lean calibration models—easy to build and maintain.
- Methods approved by regulators.
- A range of sample trays and accessories for tablets, capsules, liquids, and powders are available.
- Built-in technical controls ensure the security of your data, control access, and facilitate compliance as defined by US FDA 21 CFR Part 11 and EU Annex 11 regulations.
- Choose sustainability: The TRS100 received My Green Lab’s ACT (Accountability, Consistency, Transparency) label after independent audit for its environmental impact throughout the product lifecycle.
| Calibration Standards |
|
| Compliance |
|
| Laser Power |
|
| Laser Wavelength(s) |
|
| Resolution |
|
| Sample Tray Types (Optional) |
|
| Spectral Range |
|
Transmission Raman Spectroscopy (TRS)
Transmission Raman Spectroscopy (TRS) is an ideal method for quantitative analysis of mixtures such as tablets capsules, powders, liquids and suspensions.
Learn More- Key Literature
-
Implementing Transmission Raman Spectroscopy for Fast Content Uniformity Testing: From Feasibility Evaluation to a Validated Release Method
Dr. Meike Romer discusses why transmission Raman spectroscopy is ideal for content uniformity, how it works in conjunction with established liquid chromatography (LC) methods, and the approach that led to the implementation of TRS systems for content uniformity testing at Grünenthal Pharma.
- White Papers
- English
- 30 Mar 2021
- 1.46 MB
Quantitative Determination of Salt-Form Impurities of Warfarin at the Lowest Commercial Dose Strength using Transmission Raman Spectroscopy
An analysis and quantification of Warfarin sodium salts in intact tablets at low doses.
- Application Notes
- English
- 20 May 2019
- 1.66 MB
Quantification of Tablets Containing Multiple APIs Using Transmission Raman
The quantification of three active pharmaceutical ingredients and 2 excipients using a 9 second transmission Raman spectroscopy measurement
- Application Notes
- English
- 22 Jan 2018
- 788.16 KB
- Application Notes
- Brochures
- Flyers
- Primers
- White Papers
- FAQs
-
TRS100 & Transmission Raman FAQs
FAQs on content uniformity, polymorph and crystallinity analysis using transmission Raman spectroscopy (TRS) and the Agilent TRS100. Explore the fundamentals of TRS technology, method development, pharma QC cost saving, product compatibility, the regulatory landscape, and more.
Raman Spectroscopy Overview
Learn about Raman spectroscopy—FAQs such as what is Raman spectroscopy, how does Raman spectroscopy work, the Raman effect, as well as the advantages and disadvantages of Raman spectroscopy.
- Site Preparation Checklists
- User Manuals
More help is just a click away
If you didn't find what you need, try these resources or contact a specialist.
Agilent Transmission Raman Supporting Continuous Manufacturing of Oral Solid Drug Products
This application note explores the use of transmission Raman spectroscopy (TRS) as an at-line quantitative technique for continuous manufacturing of oral solid dose forms. TRS offers rapid, high throughput, near real-time bulk analysis of tablets and capsules.
- 02 Jul 2025
Registrations OPEN. Transmission Raman Virtual User Group Meeting 2024
Join us alongside fellow transmission Raman spectroscopy (TRS) enthusiasts from around the world. In this event, we will explore the exciting realm of TRS techniques and their successful implementation for bulk quantitative analysis in pharmaceutical applications. Discover how TRS overcomes the limitations of conventional Raman spectroscopy, enabling rapid non-invasive bulk analysis of intact pharmaceutical tablets and capsules. Register now!
- 29 Sep 2024
Agilent TRS100 Receives My Green Lab’s ACT Certification Label
The Agilent TRS100 Raman system has been certified with the ACT label in the EU, UK, and US. This clear, third-party verified information about the system’s environmental impact makes it easy for laboratories to choose products that help them achieve their sustainability goals.
- 31 Mar 2024
Read reviews on the TRS100 Raman pharmaceutical analysis system
Explore customer reviews posted on SelectScience, or write your own review.
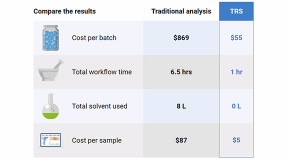
TRS Return-on-Investment Value Calculator
See how much and how soon you could save compared to your current content uniformity workflow.
Molecular Spectroscopy Pharma & Biopharma Solutions Guide
Learn about products & applications in pharma & biopharma using UV-Vis, FTIR, fluorescence, SORS & TRS technology.
Raman Spectroscopy FAQ Guide
Learn about how Raman spectroscopy works, the Raman effect, the advantages and disadvantages of Raman, and more.
Raman Spectroscopy Applications Guide
A guide to Raman spectroscopy applications using Agilent handheld and benchtop Raman analyzers.
Jerry Jin
PAT Scientist at Allergan
The analysis of 10 pharmaceutical tablets for their content uniformity takes only several minutes, as compared to one day turnaround time when using a traditional HPLC method. Method development using TRS100 for material identification and quantitative assay is generally easier than other vibrational spectroscopic techniques such as near infrared (NIR). One intrinsic advantage of TRS is its excellent specificity. Considering the unique strength of Agilent’s TRS100 in pharmaceutical analysis, I expect to see its deployment in more QC labs for product identification and assay, as well as its deployment on factory floors for real-time release testing.
View MoreVideos
- Publications
-
- Development and validation of chemometric methods for API quantification: Application of transmission Raman spectroscopy to mirabegron tablets, Higashi, Y., Yokoyama, T., Murayama, D. et al., J Pharm Innov 20, 219 (2025) Learn More
- Enabling personalised 3D printed extended-release oral drug products: Transmission Raman spectroscopy as a rapid quality control tool, Jørgensen, A. K., Griffen, J., Basit, A. W., et.al, European Journal of Pharmaceutical Sciences, 107341 (2025) Learn More
- Surface enhanced transmission Raman spectroscopy: Quantitative performances for impurity analysis in complex matrices, Horne, J., Beckers, P., Ziemons, E., et al., Journal of Pharmaceutical and Biomedical Analysis, 252 (2025) 116469 Learn More
- A non-destructive quantitative transmission Raman spectroscopy method for API in drug product in-use samples prepared in dosing vehicles, G. Doddridge, E. Hong, D.C.T. Tan et al., AAPS PharmSciTech 23, 132 (2022) Learn More
- Transmission Raman spectroscopy – Implementation in pharmaceutical quality control, Meike Römer; in 'Solid State Development and Processing of Pharmaceutical Molecules: Salts, Cocrystals, and Polymorphism', 79 (2021) 165-184 Learn More
- Analytical method development using transmission Raman spectroscopy for pharmaceutical assays and compliance with regulatory guidelines—Part II: Practical Implementation Considerations, B. Igne et al., J. Pharm. Innov., 14 2019 Learn More
- Manufacturing amorphous solid dispersions with a tailored amount of crystallized API for biopharmaceutical testing, F. Theil, H. van Lishaut et al., Abbvie Deutschland GmbH & Co., Molecular Pharmaceutics, 15 (2018) Learn More
- Calibration transfer between modelled and commercial pharmaceutical tablet for API quantification using backscattering NIR, Raman and transmission Raman spectroscopy (TRS), X. Wang et al., Journal of Pharmaceutical and Biomedical Analysis, 194 (2021) 113766 Learn More
- Analytical method development using transmission Raman spectroscopy for pharmaceutical assays and compliance with regulatory guidelines—Part I: TRS and method development, D. Andrews et al., J. Pharm. Innov., 13 (2018) Learn More
- View all spatially offset Raman spectroscopy (SORS) and transmission Raman spectroscopy (TRS) peer-reviewed publications Learn More