Headspace Sampling Fundamentals

What is Headspace?
Headspace Sampling for GC – The Basics
Headspace sampling is a type of sample introduction technique for gas chromatography (GC) and gas chromatography-mass spectrometry (GC/MS). For headspace techniques, the gas layer, or the headspace above the sample in a vial, is analyzed as opposed to withdrawing a volume from within the sample layer (Figure 1). Headspace analysis requires that the compounds of interest have high volatility, while the rest of the sample is much less or non-volatile. The technique lends itself nicely as a substitute for labor-intensive extractions when the sample types are solids, viscous liquids, blood, or medications, for example.
Headspace vials often come in larger volumes than the 2-mL vials used in many liquid sampling systems. Common offerings are 10-mL, 20-mL and 22-mL capacities. Larger vials accommodate larger sample volume and/or a larger headspace above that sample. Because headspace works best for volatile compounds, using quality vials and caps to form a tight seal is critical to a successful analysis.

Figure 1: A representation of the sample and headspace layers within a vial.
Benefits of GC Headspace
Headspace sampling offers several benefits for GC analysis.
- Compatible with virtually any matrix. The sample itself does not have to be volatile or soluble in a liquid appropriate for GC.
- Little or no sample preparation. This can lead to more reproducible results because sample prep may introduce errors into the overall workflow.
- Smaller solvent peak. In many applications, the amount of solvent introduced into the gas chromatograph is significantly less than the solvent in a liquid injection. In these cases, the solvent peak is smaller and less likely to interfere with the analytes of interest.
- Higher instrument uptime. Cleaner samples result in less maintenance to the GC inlet, column, detector, or mass spectrometer source.
- Often higher sensitivity with good precision and linearity.
Key Parts of a Headspace Sampler
Valve-and-loop headspace sampling systems, such as the Agilent 7697A and 8697 models, utilize the following parts.
- A temperature-controlled oven provides a constant temperature to incubate the sample before the GC run begins.
- A sampling probe pierces the vial and allows two conditions within the vial: 1) gas addition to increase the vial pressure and 2) sample transfer from the vial into the headspace loop.
- A heated sampling loop contains a fixed volume of sample for repeatable injections.
- A heated sampling valve allows for two separate channels of flow to decrease carryover and ensures no interruption in the carrier flow to the GC.
- A heated transfer line creates a thermally controlled channel to transfer the sample contents from the headspace sampler to the GC for analysis.
The Headspace Sampling Process
Sample is transferred to a vial suitable for headspace analysis and immediately capped to minimize loss of volatile components. Once the headspace vial is capped, these low-boiling compounds begin to migrate between the sample and the headspace layer, ultimately reaching equilibrium (Figure 2). The time to establish equilibrium is sample-dependent and is determined experimentally. This time is optimized by incubating the vial in an oven or other temperature-controlled environment.
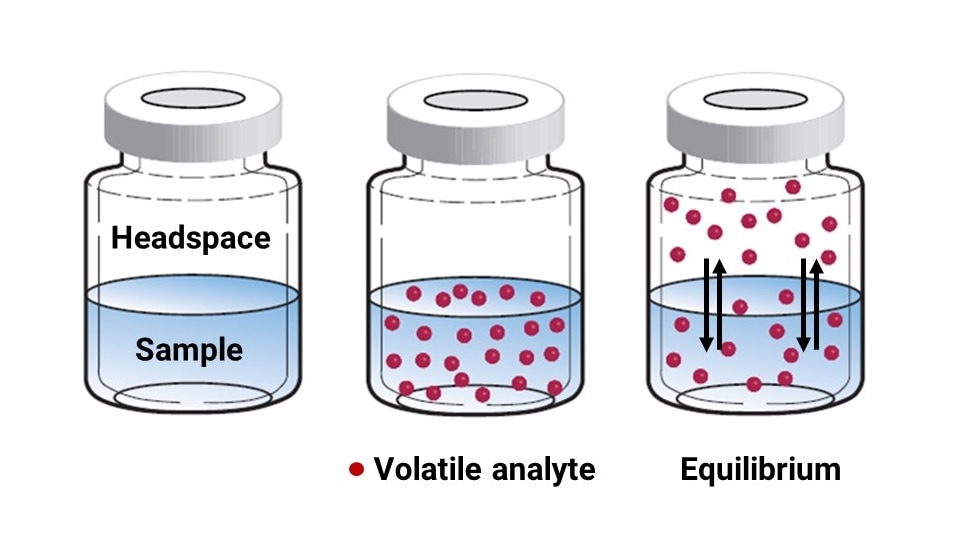
Figure 2: Depiction of volatile content moving out of the sample layer and establishing an equilibrium between the sample and the headspace.
Once equilibrium is established, the sampling process can begin. The valve and loop design (Figure 3) involves three basic steps.
- Step 1 increases the pressure within the vial by feeding in additional gas.
- Step 2 vents some of that pressure within the vial, effectively back-filling the sample loop with the gaseous phase of the sample.
- Step 3 turns the sampling valve, which injects the sample through the transfer line and into the GC inlet for analysis.
Modern headspace instruments fully automate the sampling process and, in many cases, have tools to help experimentally determine the optimal times and pressures for a particular sample type.
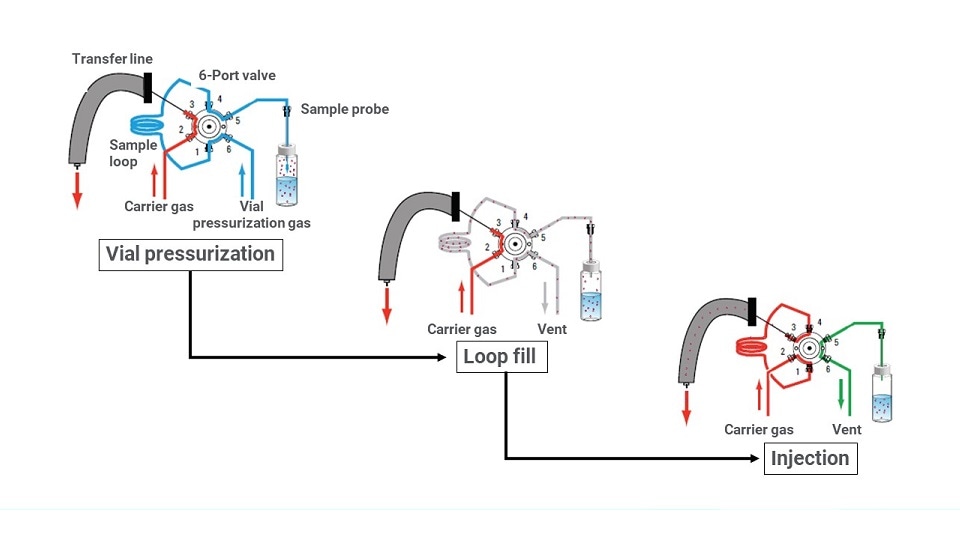
Figure 3: Three-step diagram demonstrating how valve and loop headspace samplers function.
Important Factors for Successful Headspace Analysis
The mathematical expression relating headspace concentration to GC detector response is:
A ∝ CG = C0/(K + β)
This equation shows that the area (A) found by the detector is proportional to the analyte concentration in the gas phase of the vial. That concentration (CG) is defined by dividing the concentration of the added sample (C0) by the sum of two sample-specific terms: The partition coefficient (K) and the phase ratio (β). To maximize detector response, conditions for K and β should be selected with the intent of minimizing that sum, which will increase the proportional amount of volatile targets in the gas phase of the sample. This is accomplished by optimizing the sample volume and the solubility of the analytes.
Sample volume. The phase ratio (β) is defined as the relative volumes of the gas and liquid phases in the vial (Figure 4). The phase ratio is affected by vial size and the sample volume. A best practice when optimizing phase ratio is to leave at least 50% of headspace in the vial. Therefore, using a 20-mL vial instead of a 10-mL vial allows a greater volume of sample (Figure 5). Similarly, increasing the sample volume within the same size vial also will decrease β (Figure 6)
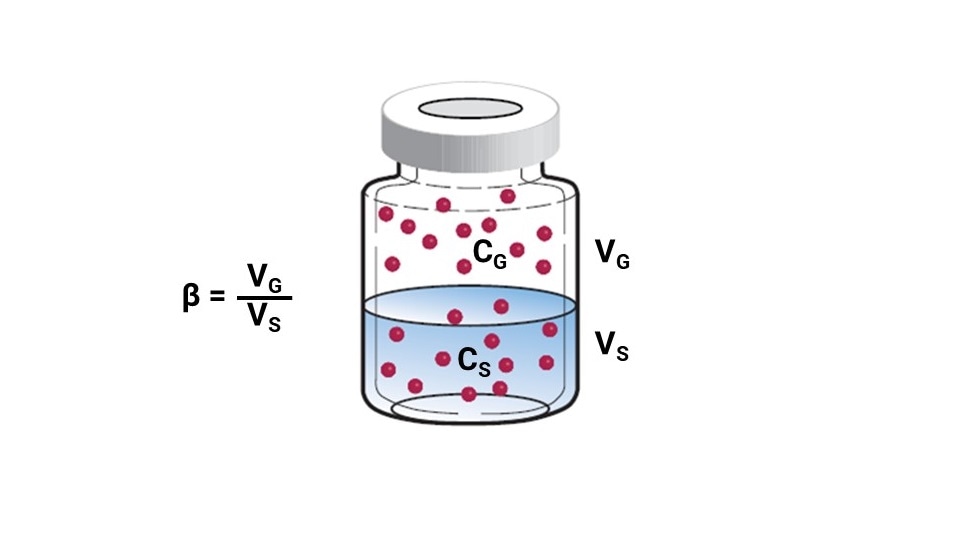
Figure 4: The phase ratio compares the volumes of the sample and the headspace inside a vial.
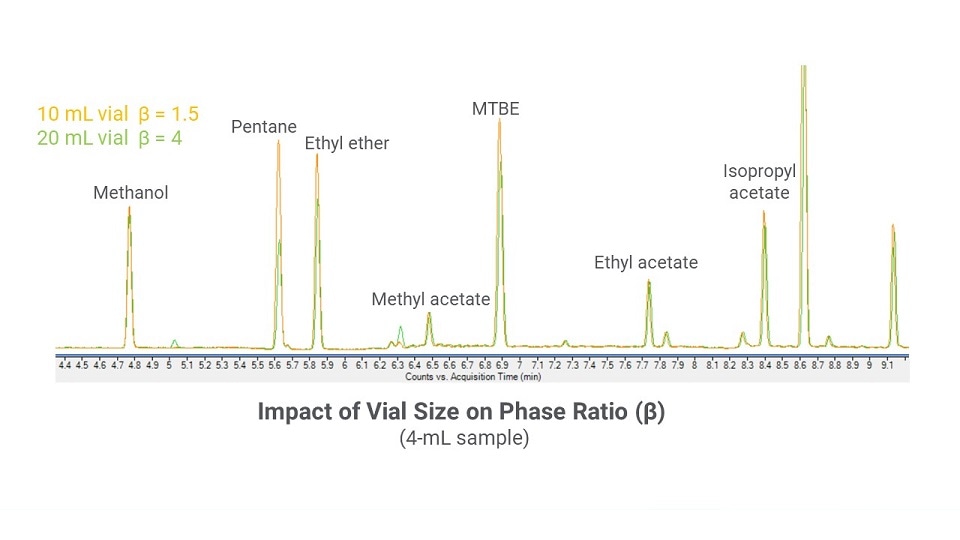
Figure 5: A chromatographic overlay of the same 4-mL sample prepared in both a 10-mL and 20-mL vial to show the impact of phase ratio on results.
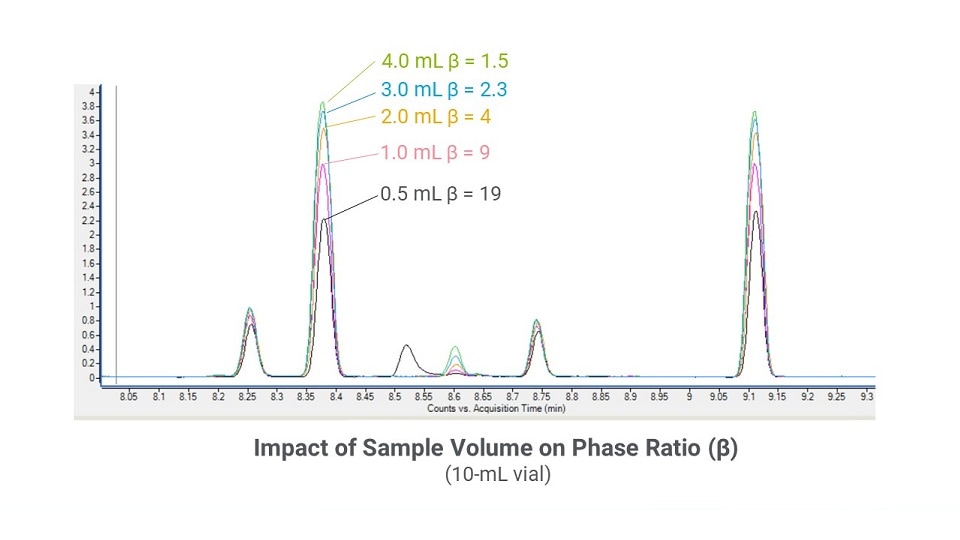
Figure 6: A chromatographic overlay showing the impact of different sample volumes within a 10-mL vial.
Sample Solubility. Headspace results are optimal when the analytes are chemically able to escape the sample and move into the headspace. This measure is called the partition coefficient (K), which is a temperature-dependent expression of sample concentration (CS) and gas phase concentration (CG). This comparison is depicted in Figure 7. In liquid samples, solvent adjustments or the addition of non-volatile salts may be essential to affect K. In solid samples, sometimes a small amount of solvent can assist in creating more favorable K values in an analysis.

Figure 7: The partition coefficient is defined by comparing the concentration of a specific compound within the sample to the concentration of the same analyte in the headspace.
To demonstrate the temperature influence on K, Figure 8 shows chromatograms for a sample that was equilibrated for 20 minutes across a range of temperatures. The higher temperature runs resulted in a higher detector response, which corresponds to more analyte in the gas phase. As an example, the K value for ethanol in water at 40 °C is ~1350. At 80 °C, the K value decreases to ~330. At some temperature setpoint, the detector response will not increase further with increasing temperature, which indicates that K has been minimized. Note that the maximum oven temperature should be kept around 20 °C below the solvent boiling point.
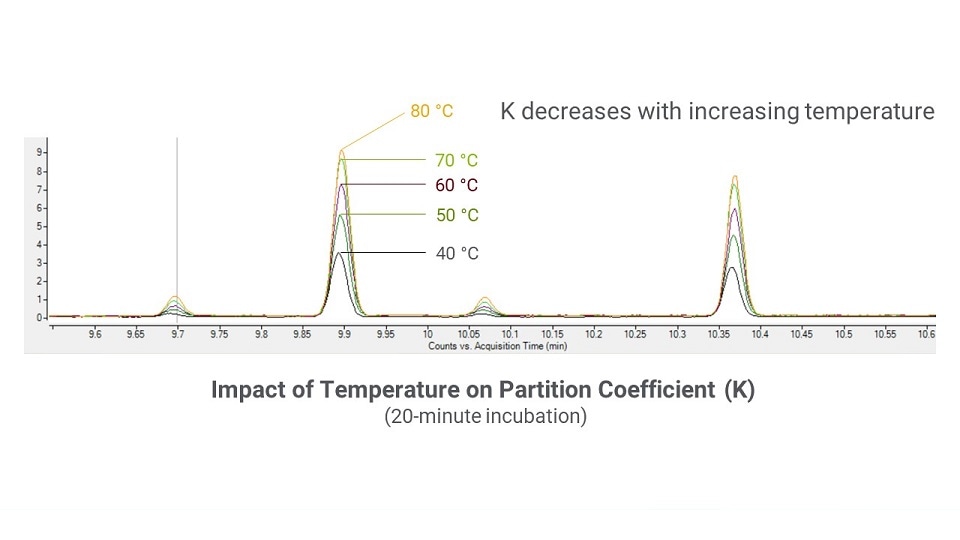
Figure 8: A chromatographic overlay of sample replicates equilibrated at different temperatures for 20 minutes.
Other headspace parameters can affect detector response. Settings such as equilibration time, vial shaking, sample loop volume, and sample loop pressure can improve the consistency and repeatability of the analysis. For a more complete overview of these and other factors that can impact headspace analysis, watch this webinar.
Using Multiple Headspace Extraction to Improve Accuracy
The headspace sampling process described above is a single extraction per vial. However, when interfering matrices are present in the headspace or in cases where a calibration standard cannot be made with the same matrix composition, quantitation can be inaccurate. Multiple Headspace Extraction (MHE) involves a series of sampling cycles using the same vial. The sample is pressurized and an aliquot is taken from the headspace and injected into the GC. This is repeated multiple times to obtain the final result. Multiple Headspace Concentration (MHC) is the same as MHE but instead of injecting into the GC after every headspace aliquot is taken, the sample is concentrated in the GC inlet using a cryo trap.
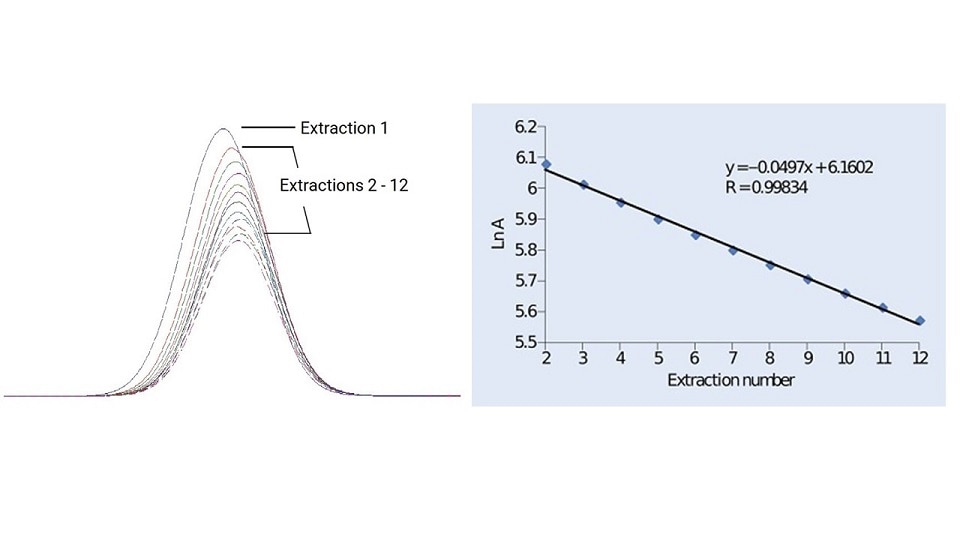
Figure 9: Multiple headspace extractions.
Common Applications for GC Headspace
Residual Solvents
The United States Pharmacopeia (USP) method 467 is a common headspace application. It detects and measures solvents from the pharmaceutical manufacturing process that may be present in over-the-counter and prescription drugs to ensure they meet regulatory guidelines and are safe for consumers.
With the legalization of medical marijuana in many states and countries, residual solvents analysis also is being used for cannabis products.
- Analysis of USP Method <467> Residual Solvents on the Agilent 8890 GC System.
- Novel Residual Solvents Analysis of Cannabinoid Products with the Agilent Headspace-GC/MS System
Blood Alcohol Analysis
Another widely used application of headspace gas chromatography is the determination of ethanol content in blood. Because these cases often go to trial, accurate and defensible data are critical.
- Analysis of blood alcohol concentration with an Agilent Intuvo 9000 GC system
- Blood Alcohol Analysis with the Integrated Agilent 8697 Headspace Sampler on 8890 GC-Dual FID System
Volatiles in Environmental Samples
Headspace-GC is used by environmental labs to analyze soil and water samples for the presence of volatile compounds that may pose a health risk to humans and animals.
- Fast Volatile Organic Compound Analysis of Drinking Water Using the Agilent 8697 Headspace Sampler in Tandem with Intuvo 9000 GC and 5977B GC/MSD
- Volatile Organic Compounds Analysis in Soils and Sediments Using the Agilent 8697 Headspace Sampler
Flavor Compounds in Foods and Beverages
Chemists use headspace sampling with gas chromatography to determine the volatile compounds in foods and beverages. Characterizing and quantitating them are critical to ensure product quality and consistency.
There are many other uses for headspace analysis, such as solvents and residual monomers in polymers; transformer oil gas analysis; contaminants in cleaning products, cosmetics, and personal care products; sterilization by-products in medical devices; and more.
Additional Information
7697A Headspace Sampler Method Development Wizards
Book: Kolb, B.; Ettre, L. Static headspace-gas chromatography theory and practice, 2nd ed.; John Wiley and Sons, Incorporated, 2006.
Related Products