Access Agilent eNewsletter, August 2013
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Coupling the Agilent 7697A Headspace Sampler to the 7890G GC and 5977A MSD provides sensitive, robust and reproducible analysis of residual solvents by USP <467>
By Craig Marvin
Agilent GPD Solutions Business Manager
Producers of active pharmaceutical ingredients (APIs), excipients and formulations use a number of chemical solvents to improve purity, develop specific characteristics, and increase yield. Because these solvents have toxic and environmentally hazardous properties – and can be very difficult to remove from finished products – regulatory bodies such as the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), United States Pharmacopeia (USP), and European Pharmacopeia (EP) have all defined limits for residual solvents in pharmaceutical formulations.
Based on risk to human health, USP <467> places Residual Solvents into one of three classes [1]:
Class 1 Residual Solvents: Solvents to be avoided
- Known human carcinogens
- Strongly suspected human carcinogens
- Environmental hazards
Class 2 Residual Solvents: Solvents to be limited
- Nongenotoxic animal carcinogens, or possible causative agents of other irreversible toxicity, such as neurotoxicity
- Solvents suspected of other significant but reversible toxicities
Class 3 Residual Solvents: Solvents with low toxic potential
- Solvents with low toxic potential to humans; no health-based exposure limit required
When coupled to the Agilent 7697A Headspace Sampler, the Agilent 7890B Gas Chromatograph and 5977A Mass Spectrometer deliver accurate and reliable analyses of Class 1, Class 2A, and Class 2B OVIs per USP <467>.
Repeatable performance satisfies USP <467> limit concentration requirements
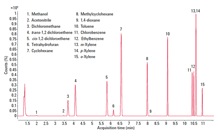
To evaluate the suitability of the analyzer for USP <467>, we prepared class 1, class 2A, and class 2B residual solvents at their limit concentrations in purified water (shown in Figures 1 – 3). We performed the analysis according to USP <467> Procedure A (Identification and Limit Testing) using an Agilent 7697A HS/7890B GC/5977A MSD system configured to provide a good compromise between resolution, speed, capacity, and ease-of-use.
 Enlarge
Enlarge
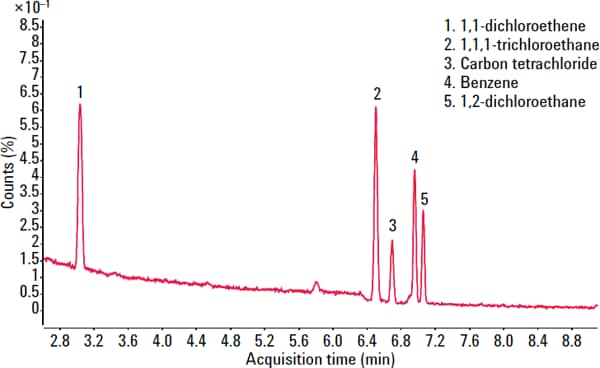
Figure 1. Total ion chromatogram (TIC) for the Class 1 residual solvents.
 Enlarge
Enlarge
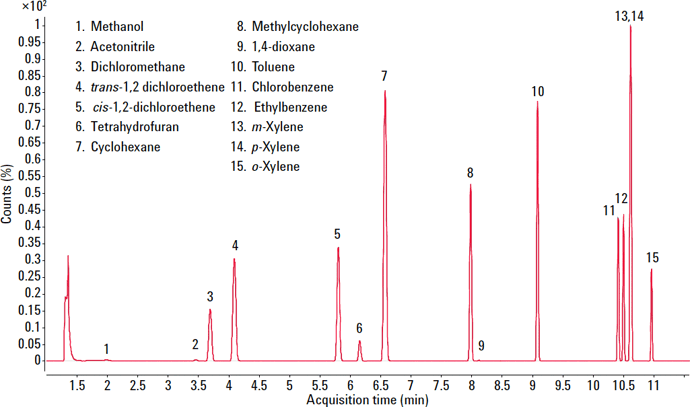
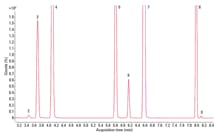
Figure 2. Total ion chromatogram (TIC) for class 2A solvents.
 Enlarge
Enlarge
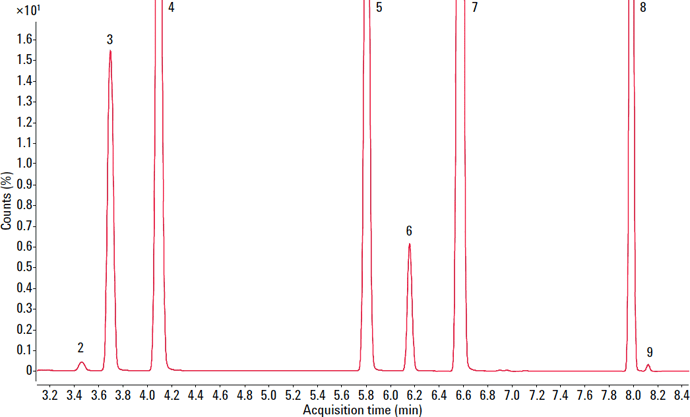
Figure 3. Zoom of total ion chromatogram (TIC) for the Class 2A solvents (See figure 2 for peak numbers) information.
Electronic Pressure Control (EPC) controlled sampling in the headspace provided concise and reproducible sampling with minimal carryover. The use of controlled venting in the Agilent 7697A gave the user increased flexibility, better repeatability, and – depending on the analyte Electronic Pressure Control (EPC) k (partition coefficient) value – enhanced sensitivity.
For our analysis [6], we achieved good sensitivity and reproducibility by injecting through a multimode inlet with a split ratio of 20:1. Split injection provided a flow sufficient to quickly flush the loop and transfer the sample to the column in a tight band. We accomplished separation using an Agilent VF-624MS and performed data analysis with the Agilent MassHunter Workstation software.
Table 1 summarizes the Scan RSD for all solvent classes and Selected Ion Monitoring (SIM) RSD for Class 2A solvents. The system produced excellent reproducibility – below 2.5% RSD – for the majority of compounds. (Compounds with low k’s generally demonstrated higher RSD due to variability in sample preparation.) Preparation of samples in other solvents such as dimethylsulfoxide (DMSO), dimethylacetamide (DMAC), 1,3 dimethyl-2-imidazolinone (DMI) or mixed such as DMSO/water may change peak response. However, analytical reproducibility for samples prepared in these solvent systems should demonstrate RSD equal to, or better than, the RSD values obtained from samples in the aqueous diluent used in this study.
Compound |
USP Limit (ppm) |
Scan RSD (%) |
SIM RSD (%) |
|
|---|---|---|---|---|
Class 1 |
n=8 |
|||
1,1-dichloroethene |
8 |
0.9 |
||
1,1,1-trichloroethane |
1500 |
1.9 |
||
Carbon tetrachloride |
4 |
1.5 |
||
Benzene |
2 |
0.7 |
||
1,2-dichloroethane |
5 |
0.9 |
||
Class 2A |
n=10 |
|||
Methanol |
3000 |
2.8 |
2.4 |
|
Acetonitrile |
410 |
3.3 |
2.3 |
|
Dichloromethane |
600 |
2.5 |
2.2 |
|
Trans 1,2--dicloroethene |
1870 |
2.4 |
2.2 |
|
Cis 1,2-dichloroethene |
1870 |
2.1 |
2.1 |
|
Tetrahyrofuran |
720 |
3.0 |
2.2 |
|
Cyclohexene |
3880 |
2.7 |
1.3 |
|
Methylcyclohexane |
1180 |
4.3 |
1.6 |
|
1,4-dioxane |
380 |
2.6 |
2.3 |
|
Toluene |
890 |
0.7 |
2.0 |
|
Chlorobenzene |
360 |
1.9 |
2.1 |
|
Ethylbenzene |
2170 |
1.9 |
2.1 |
|
m-xylene, p-xylene |
2170 |
2.1 |
1.8 |
|
o-xylene |
2170 |
2.1 |
1.8 |
|
Class 2B |
n=9 |
|||
Hexane |
290 |
3.2 |
||
Nitromethane |
50 |
3.8 |
||
Chloroform |
60 |
2.5 |
||
1,2-dimethoxyethane |
100 |
2.7 |
||
Trichloroethene |
80 |
2.5 |
||
Pyridine |
200 |
3.9 |
||
2-hexanone |
50 |
2.4 |
||
Tetralin |
100 |
2.5 |
||
Table 1. Residual solvent Class 1, Class 2A, and Class 2B repeatability. (Scan data shown for all classes and SIM data for class 2A. Prepared at limit concentrations in aqueous diluent.)
A Residual Solvent Analyzer configured with the Agilent 7697A HS/7890B GC/5977A MSD produces outstanding repeatability for the analysis of residual solvents per USP <467>. Incorporating the MSD creates a powerful analytical tool for investigating Organic Volatile Impurities (OVI) content in APIs, excipients and product – especially for identifying unknown residues in drug discovery and during production scale up.
The Agilent 7697A HS/7890B GC/5977A MSD configuration is especially useful in drug discovery and process scale up where identification of unknowns is critical. The methods described in this article provide the basis for developing internal procedures for the analysis of residual solvents.
References:
- USP 32-NF 27, General Chapter USP <467> Organic volatile impurities, United States Pharmacopeia. Pharmacopoeia Convention Inc., Rockville, MD, 8/2009
- Albert E Gudat and Roger L.Firor, “Improved Retention Time, Area Repeatability, and Sensitivity for Analysis of Residual Solvents”, Agilent Application Note 5989-6079EN
- Roger L. Firor, “Analysis of USP<467> Residual Solvents with Improved Repeatability Using the Agilent 7697A Headspace Sampler”, Agilent Application Note 5990-7625EN
- Bart Tienpont, Frank David, Pat Sandra, and Roger L. Firor, “Analysis of USP<467> Residual Solvents using the Agilent 7697A Headspace Sampler with the Agilent 7890B Gas Chromatograph”, Agilent Application Note 5991-1834EN
- Roger L. Firor, “Optimizing Vial Pressurization Parameters for the Analysis of USP<467> Residual Solvents Using the 7697A Headspace Sampler”, Agilent Application Note 5990-9106EN
- Roger L. Firor and Mke Szelewski, “Applying the Agilent 5977A MSD to the Analysis of USP<467> Residual Solvents with the 7697A Headspace Sampler and 7890B GC”, Agilent Application Note 5991-2079EN
>> Update My Profile | Subscribe to Access Agilent | Article Directory