Access Agilent eNewsletter, October 2014
>> Update My Profile | Subscribe to Access Agilent | Article Directory
ICP-MS supports Quality by Design methods to determine elemental impurities in pharmaceutical manufacturing
By Ed McCurdy
Agilent ICP-MS Product Marketing
Rugged manufacturing and analytical processes are essential to ensuring consistency across a worldwide pharmaceutical manufacturing and distribution system. Recently, the pharmaceutical industry has increased its focus on developing more reliable, consistent, and accurate methods for ensuring product quality and safety. Regulatory bodies including the European Medicines Agency (EMA) and the United States Food and Drug Administration (U.S. FDA) are coordinating the development of analytical systems design and methodology to support the same aims.
The control of inorganic contaminants in pharmaceutical products is also receiving attention, as the potential impact of catalyst residues, toxic trace elements, and other metals becomes better understood. The International Conference on Harmonization (ICH) has published guidelines (ICH-Q3D) that cover the control of Elemental Impurities in pharmaceutical products. In parallel, the U.S. Pharmacopeia (USP) is introducing new General Chapters <232> and <233> to replace the existing USP<231> (heavy metals limit test), which is no longer considered suitable for assessing product quality and safety. USP<231> delivers inherently variable results as it is based on a subjective color comparison. Also, the method does not give specific quantitative results for each element, provides no information on catalyst residues, and the sample preparation leads to low recoveries for volatile elements, such as Hg.
The FDA’s recent draft guideline on Analytical Procedures and Method Validation [1] also recommend that current methodologies and instrumentation are assessed as part of a process of continuous improvement, and replaced with modern alternatives where such new technologies offer improved data quality. Because Quality by Design (QbD) seeks to ensure consistency in a manufacturing process, analytical methodology, product, or procedure by identifying, understanding, and controlling the sources of variability, its principles are increasingly being adopted across the pharmaceutical industry. QbD satisfies the complementary aims of regulatory compliance and manufacturing quality control.
 Enlarge
Enlarge
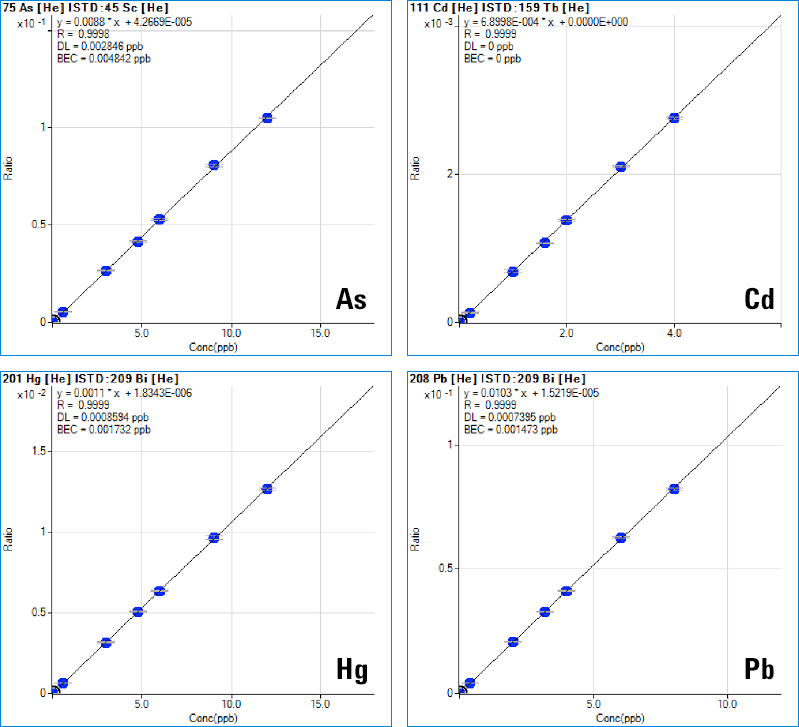
Figure 1. Linear calibrations for specific, quantitative analysis of the “Big Four” toxic trace elements in pharmaceutical products.
ICP-MS with auto-optimization and method automation delivers consistent operation
The proposed new USP and ICH methods use modern sample preparation procedures (microwave digestion) and instrumental analysis techniques – inductively coupled plasma mass spectrometry (ICP-MS), and optical emission spectroscopy (ICP-OES) – that provide specific, accurate, and traceable quantitative data for all the elements of interest for both manufacturing quality control and product safety.
The Agilent 7900 ICP-MS supports the principles of QbD by providing accurate quantitative measurement of all regulated elements in a wide range of pharmaceutical materials. Because of the very high temperature of the ICP ion source, ICP-MS offers high and consistent ionization efficiency (leading to high sensitivity) and is largely free from matrix effects, so linear calibrations based on simple synthetic calibration standards can be used for a wide range of sample matrices (Figure 1). The ICP-MS MassHunter software platform includes a new Method Automation function to simplify method development, making it easier for users to develop reliable methods for their particular sample types. This not only simplifies method setup and routine analysis, but also ensures consistent operation among users with different levels of experience. Method ruggedness (consistency of results between batches run on different days, on different instruments, or by different operators) is a key part of the performance testing defined in USP<233>, and a central principle of QbD.
 Enlarge
Enlarge
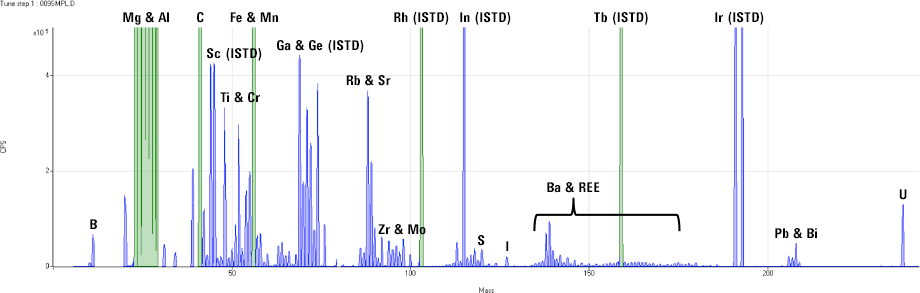
Figure 2. ICP-MS full mass screening acquisition for a commercial antacid sample.
Unequivocal identification
Inorganic mass spectrometry offers a degree of certainty that few alternative techniques can match. All naturally occurring elements – except indium [In] – have at least one isotope that is free from overlap by an isotope of another element, so target elements can be identified with a high degree of confidence. Where interferences do arise, they are usually polyatomic overlaps, which means several atoms combine to form a [usually] di-atomic molecular ion at the same mass as the analyte. These polyatomic ions can easily be separated from the analyte ions using collision/reaction cell technology. Where several isotopes are available for an analyte, quantitative results can be calculated independently based on the two (or more) isotopes, giving unequivocal confirmation of the analyte identity and concentration based on the secondary or “qualifier” ion result. Furthermore, the simple mass spectra of ICP-MS are ideal for screening analysis during method development and investigative work (Figure 2). In a single measurement, taking around 3 minutes or less per sample, virtually every element can be positively identified and an approximate concentration calculated.
Find more information on Agilent solutions to support QbD implementation in our recently published primer (5991-2166EN) [2].
References
- U. S. Food and Drug Administration/Center for Drug Evaluation and Research/Center for Biologics Evaluation and Research. Analytical Procedures and Methods Validation for Drugs and Biologics: Draft Guidance for Industry, Washington, DC (2014).
- Gain Greater Confidence. Agilent Solutions for Quality-by-Design Implementation in Pharmaceutical Development, Agilent Primer, 5991-2166EN.
Agilent ICP-MS Journal
Are you interested in trace metals analysis and eager to keep up-to-date with the latest developments in the field of ICP-MS? Then we invite you to take a closer look at Agilent’s dedicated ICP-MS Journal. Published four times a year and available as a PDF, you can view the latest issue of the ICP-MS Journal, plus all previous copies, by visiting the ICP-MS Journal Archive.
To receive a personal copy of the ICP-MS Journal direct to your inbox, please sign up here.
>> Update My Profile | Subscribe to Access Agilent | Article Directory