PD-L1 IHC 28-8 pharmDx for Esophageal Carcinoma (EC)

nivolumab + chemotherapy or nivolumab + ipilimumab

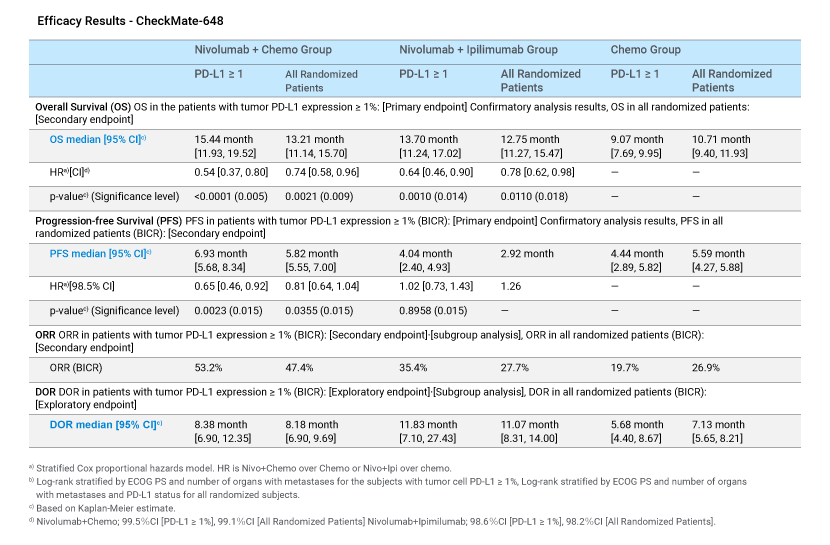
- Phase 3 randomized, open-label study in chemotherapy-naive patients with unresectable advanced or recurrent esophageal cancer, to compare nivolumab + chemotherapy or nivolumab + ipilimumab versus chemotherapy alone.
- The study results highlight overall survival (OS) and progression-free survival (PFS) benefit from nivolumab + chemotherapy, and OS benefit from nivolumab + ipilimumab in esophageal cancer patients with tumor cell PD-L1 ≥ 1%.
PD-L1 IHC 28-8 pharmDx is MHLW-approved and fully validated with analytical performance having met all pre-determined acceptance criteria for sensitivity, specificity and precision.
| Selected analytical validation parameters | Description |
|---|---|
| Specificity |
|
| Sensitivity |
|
| Reproducibility |
|
OA = Overall Agreement
| Product | Code |
|---|---|
| PD-L1 IHC 28-8 pharmDx | SK005 |
|
Required but not included in kit: Autostainer Link 48 EnVision FLEX Wash Buffer, 20x EnVision FLEX Hematoxylin (Link) PT Link PT Link Rinse Station |
AS480 K8007 K8008 PT100 / PT200 PT109 |
- Doki Y., Ajani. J.A., Kato K., et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma.
N. Engl. J. Med. 2022, 386(5), 449-462. - PD-L1 IHC 28-8 pharmDx Instructions for Use.
- Ipilimumab package insert.
- Nivolumab package insert.
Intended Use
To measure the proportion of PD-L1 expression in cancer tissue or cells
- Indication as an aid for proper administration of nivolumab [recombinant] in non-small cell lung cancer [NSCLC] patients,
head-and-neck cancer patients, gastric cancer patients, and esophageal cancer patients. - Indication as an aid for proper administration of nivolumab [recombinant] and ipilimumab [recombinant] combination in melanoma patients.
Important Basic Precautions
It is desirable to measure PD-L1 expression by PD-L1 IHC 28-8 pharmDx in determining whether or not the following drugs can be administered.
- Nivolumab [recombinant] for the patients with non-squamous NSCLC patients or head-and-neck cancer patients who have been treated with chemotherapy previously.
- Combination therapy of nivolumab [recombinant] and chemotherapy for NSCLC patients or gastric cancer patients who have not been treated with chemotherapy.
- Combination therapy including nivolumab [recombinant] for esophageal cancer patients who have not been treated
with chemotherapy. - Combination therapy of nivolumab [genetic recombinant] and ipilimumab [genetic recombinant] for melanoma patients and esophageal cancer patients.
If it is not possible to measure PD-L1 expression using PD-L1 IHC 28-8 pharmDx, refer to the packaging insert of the drugs, and appropriately judge the adequacy of administration.




