Non-Small Cell Lung Cancer (NSCLC)
PD-L1 IHC 28-8 pharmDx
IVD

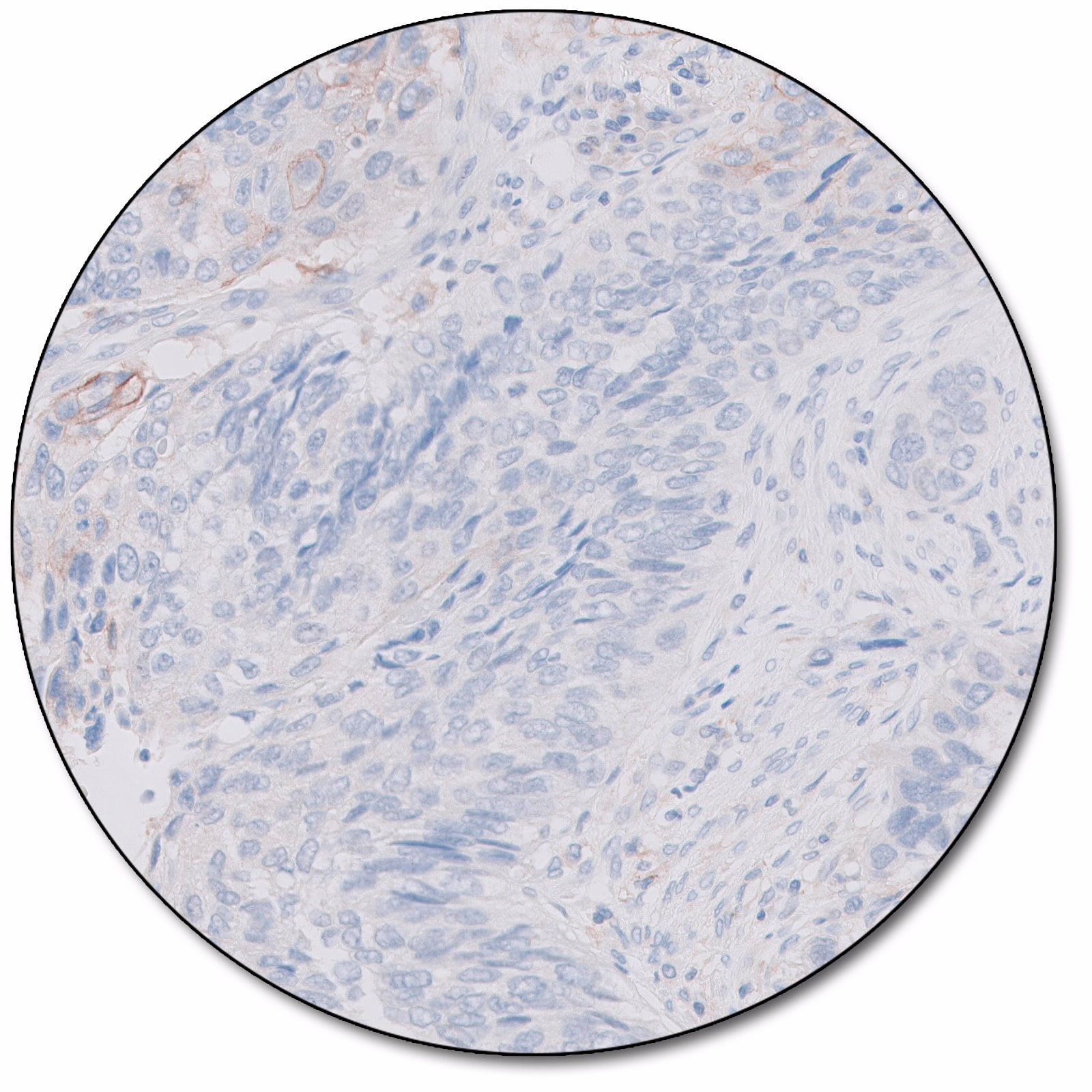
PD-L1 IHC 28-8 pharmDx is a qualitative immunohistochemical assay using Monoclonal Rabbit Anti-PD-L1, Clone 28-8 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-squamous non-small cell lung cancer (nsNSCLC), non-small cell lung cancer (NSCLC), melanoma, head and neck cancer, gastric cancer, and esophageal cancer, tissues using EnVision FLEX visualization system on Autostainer Link 48.
It is desirable to measure PD-L1 expression by 28-8 kit in determining whether or not the following drugs can be administered.
・Nivolumab [recombinant] for the patients with non-squamous NSCLC or head-and-neck cancer patients who have been treated with chemotherapy previously.
・Combination therapy of nivolumab [recombinant] and chemotherapy for NSCLC patients or gastric cancer patients who have not been treated with chemotherapy.
・Combination therapy of nivolumab [recombinant] and ipilimumab for melanoma patients.
If it is not possible to measure PD-L1 expression using 28-8 kit, refer to the packaging insert of the drugs, and appropriately judge the adequacy of administration.
Interpretation training is now available for PD-L1 IHC 28-8 pharmDx. To learn more, visit PD-L1 IHC 28-8 pharmDx eLearning.
PD-L1 IHC 28-8 pharmDx is subject to an exclusive trademark license to Agilent Technologies, Inc. OPDIVO® is a trademark owned by ONO in Japan, Korea and Taiwan and by Bristol-Myers Squibb in the rest of the world. YERVOY® is a trademark owned by Bristol-Myers Squibb.
Reg. Status: IVD