Virology
January 2021
A critical component of virology studies and infectious disease research is accurate determination of viral titers. There are several well-established methods for measuring viral titers, all of which rely on serially diluted virus samples being added to an evenly distributed monolayer of cells permissive to viral infection. Choosing the most appropriate viral titer method for a given virus should consider the properties of the virus itself and the desired throughput and reagents availability. When working with lytic viruses, viral titers can be determined using either the plaque assay or the TCID50 assay. For lysogenic viruses, the Focus-Forming Assay is the gold standard for determining viral titers, although the TCID50 assay may also be an effective format.
Regardless of the selected format, BioTek automated imaging systems and liquid handlers provide optimized solutions for measuring viral titers. Efficient image capture and analysis protocols enable accurate and reproducible quantitative results for a broad range of virology applications.
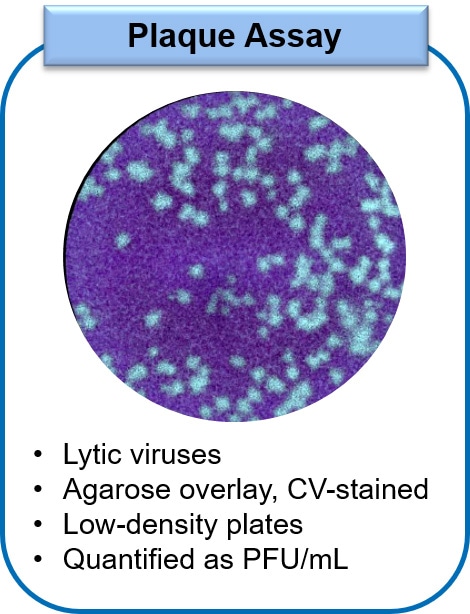
The Plaque Assay relies on the ability to count individual clearings in the monolayer, termed plaques, that arise from viral lysis of infected cells. The plaque assay procedure consists of adding virus of a known dilution from a stock sample onto the cell monolayer, followed by the addition of an agarose overlay and then fixation and counterstaining of the cells. Typically, the plaque assay is conducted in low-density microplates due to the manual manipulation of the samples that is required, limiting throughput. However, as the assay is designed such that each clearing or plaque should arise from a single virion at appropriate dilutions, this method is considered highly accurate for determining viral titers. The determined virus concentration is expressed as plaque forming units (PFU) per mL.
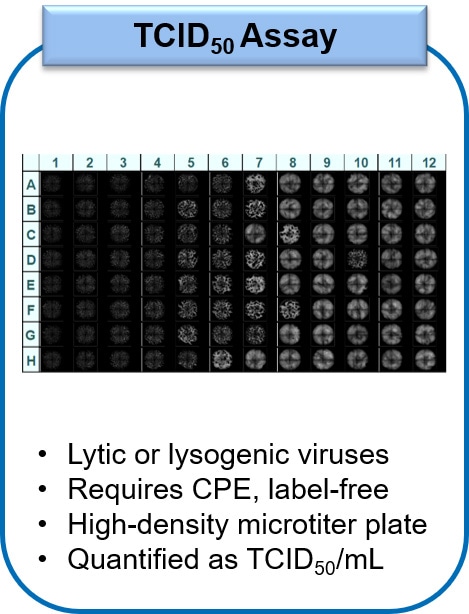
The TCID50 Assay is a flexible method for conducting viral titration that can be used for both lytic or lysogenic viruses. The TCID50 assay relies on the detection of viral-induced cytopathic effects (CPE), to determine the presence of infectious virus in each well. This assay format does not require a labor-intensive overlay or counterstain, thus the format is more forgiving and requires less practice and skill to perform appropriately compared to the plaque assay. Additionally, this assay can be performed in higher density microtiter plates, typically 96- to 384-well plates, greatly increasing throughput for viral titration. Calculation of viral titers relies on mathematical calculation based on the percentage of infected replicative wells, therefore this method is not considered as accurate as other viral titration methods, with titers expressed as TCID50/mL.
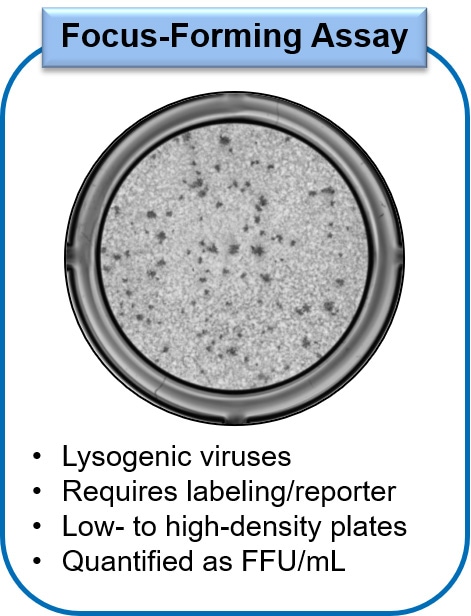
Using the Focus Forming Assay, viral titers are determined by two different methods depending on available reagents. In one method, the infected monolayers are fixed and immunostained using a virus-specific primary antibody, followed by detection with a labeled secondary antibody. Alternatively, if there is a fluorescent reporter strain of the virus available, infected cells can be imaged and the number of fluorescing infected cells or foci can be determined by image analysis. In both methods, each focus of viral infection should arise from a single infectious virion at appropriate dilutions, contributing to the high accuracy of the Focus Forming Assay at determining viral titers. This assay can be performed in either low- or high-density microtiter plates, with the throughput on the method used. The determined concentration of virus is expressed as focus-forming units or FFU per ml.
Featured Applications
Automated Viral Plaque Assay Workflow Using the Cytation Cell Imaging Multi-Mode Reader
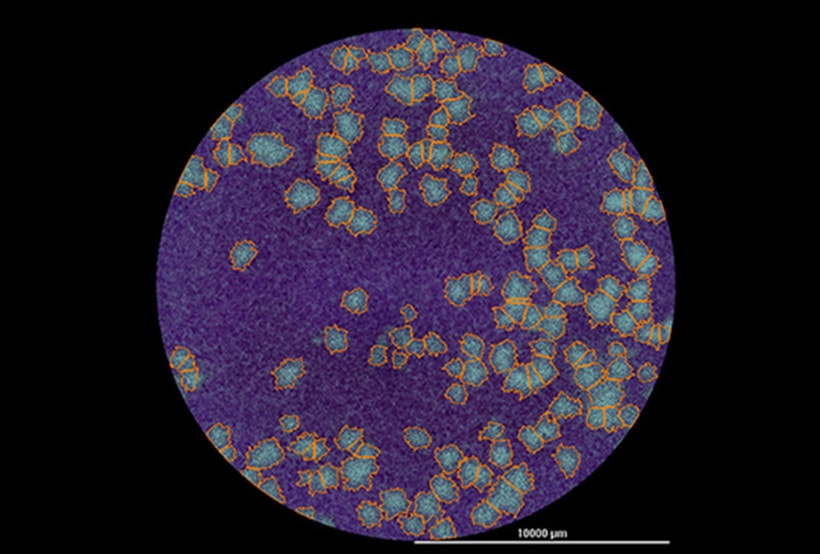
Plaque assays remain the standard method for determining viral titers for lysogenic viruses. The assay relies on determining the number of plaque-forming units (PFU) created in a monolayer of virus-infected cells. Plaques form when a virus-infected cell Iyses, leading to subsequent cycles of infection and lysis of neighboring cells. The resulting cellular dead zone, or plaque, surrounded by non-infected cells is identified using a counterstain, typically a crystal violet solution, and optical microscopy or visual inspection.
Monitoring Viral Infection of Mammalian Cells using Digital Fluorescence Microscopy
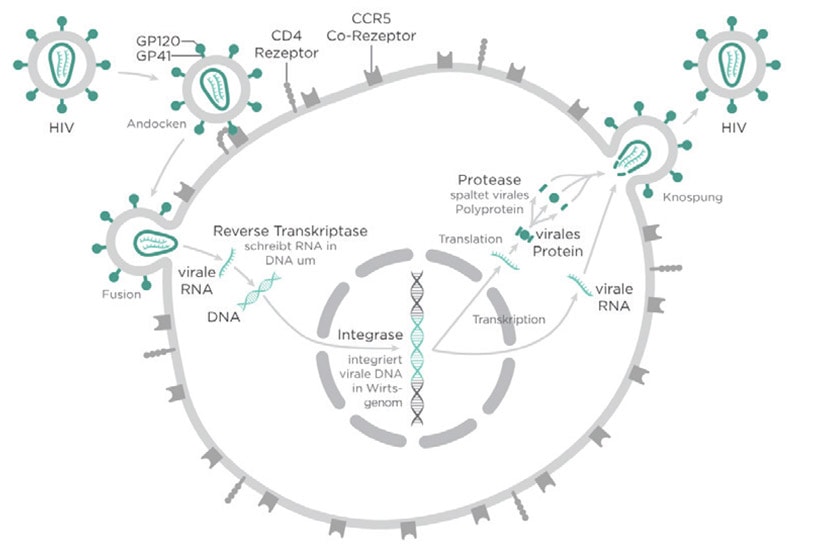
Virus quantification involves the determination of the number of viruses in a specific volume. One method to determine a viral titer is the endpoint dilution assay, which determines a 50% tissue culture infective dose (TCID50). This method involves infecting tissue culture cells and is useful for viruses that do not form plaques. Here we demonstrate the ability of the Cytation 5 Cell Imaging Multi-Mode Reader and Gen5 Microplate Reader and Imager Software to determine infection levels of HIV with HeLa cells in culture.
Product Spotlight
Cytation 7 Cell Imaging Multi-Mode Reader
Cytation 7 Cell Imaging Multi-Mode Reader combines automated digital upright and inverted widefield microscopy with monochromator-based multi-mode microplate reading. The inverted microscope provides 1.25x to 60x magnification in fluorescence, brightfield and color brightfield, while the upright microscope enables other common applications including ELISpot, slide scanning and ROI detection.
MultiFlo FX Multi-Mode Dispenser
MultiFlo FX Multi-Mode Dispenser is a versatile tool for liquid handling workflows. MultiFlo FX automates fast dispensing and washing, gentle media exchange for non- or loosely-adherent cell based assays, and dispensing into individual wells. Its unique Parallel Dispense design allows up to four independent reagents to be dispensed in parallel without carryover.
MultiFlo FX can incorporate one or two peristaltic dispense pumps, plus two syringe dispense pumps, plus an available microplate wash module. The unique random access dispense (RAD) module can automatically dispense varying volumes into discrete wells of a 96- or 384-well plate for normalization protocols, or for building serial dilutions automatically. The new patented automated media exchange module (AMX) automates critical steps in spheroid and non-adherent cell based assays – all one on compact platform.
Tek Tips
Best practices for achieving uniform, fully confluent monolayers for any viral titration method
In the virology field, microplate-based cell culture monolayers are used for many general virology techniques, including screening for viral infection, propagating viral stocks, and titering virus stocks and samples. Steps taken to promote an even distribution of cells across the microplate culture surface will improve the accuracy and reproducibility of assay results in general, but are particularly important for successfully conducting the plaque assay.
1. Prepare cell suspension
- Determine the total concentration of cells and final volume of media required to seed all required microplates at determined seeding density
- Total Cells = (#cells/well) x (#wells)
- Total Volume= (µL media/well) x (#wells)
- Prepare cells for seeding, spinning down the total number of cells and thoroughly resuspending in the specified total volume
2. Cell Seeding
- Ensure that cells remain suspended evenly in solution during cell seeding process
- In high density microplates, seed cells with a multichannel pipette, repeat pipettor, or use a BioTek automated cell dispenser, such as the MultiFlo FX Microplate Dispenser or MicroFill Dispenser
- Manual seeding procedure
- It is best to seed cells into the center of each well and not to the side
- Since cells have been resuspended in the final volume of media required for cell seeding, it is not necessary to add additional media to each well following seeding
- Manual seeding procedure
- Immediately following cell seeding, carefully rock each plate in an X-Y direction for 1-2 minutes without swirling. This distinct X and Y rocking is key to an even monolayer distribution. Any swirling will deposit the majority of cells at the edges of the well.
- Allow microplates to sit at room temperature for approximately 20 min before transferring to the culture incubator. This step is critical for cells to settle down uniformly across the plate after rocking.
Optimizing inoculation of microplate-based cell culture monolayers
1. Inoculum Adsorption
- The spent media must be removed prior to adding the inoculum
- Do not remove all of the media, leave just enough media to cover the monolayer to prevent the monolayer from drying out
- Dried areas of monolayer will easily peel during subsequent processing steps
- Gently remove spent media either manually or using optimized slow and gentle aspiration settings on a BioTek automated liquid handler
- Manual removal procedure
- Tip plates to the side and slowly remove most of the media using a large bore pipette tip
- If using a vacuum, place a large bore pipette tip over the tip and use a low setting to prevent monolayer disruption
- Do not allow the tip to contact the monolayer
- Manual removal procedure
2. Inoculum Removal
- Apply the gentle aspiration techniques described above to remove the inoculum following incubation
- If wash steps are required, gently add pre-warmed solution to the monolayer either manually or using gentle dispense settings for automated liquid handling
Virology Resources
For Research Use Only. Not for use in diagnostic procedures.


