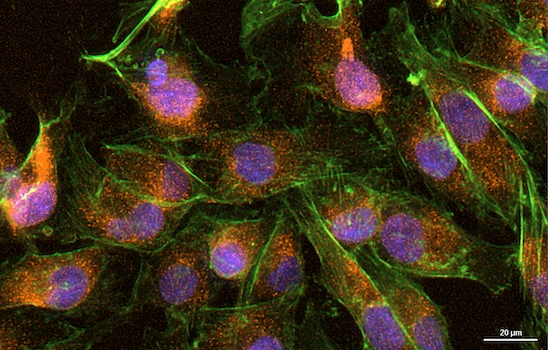
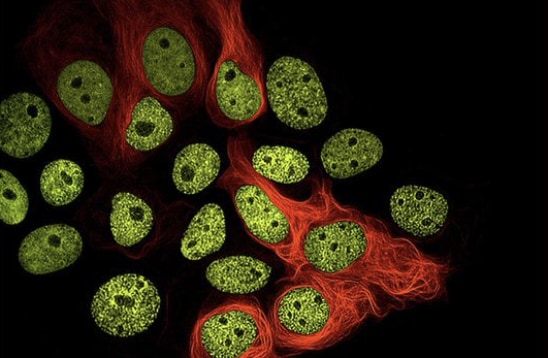
Proximity Assays
March 2023
Biomedical research requires that numerous analytes be quantitated, often in large numbers of experimental samples. Towards that end, different homogeneous proximity assays have been developed to quantitate specific analytes from a complex sample matrix without the need for wash steps to remove unwanted contaminates. Eliminating the necessity to remove unbound contaminates not only saves considerable amounts of time, but also reduces the amount of instrumentation needed to automate the assays. These assays share a common feature in that signal is only generated in the presence of analyte, resulting in low background signal and excellent S/B ratios. As the name implies, this is accomplished by bringing two molecules together in close enough proximity such that an energy transfer can take place, with the analyte serving as the bridge. Typically, antibodies serve as the means for analyte specificity. These features make them the preferred assay type for many screening experiments.
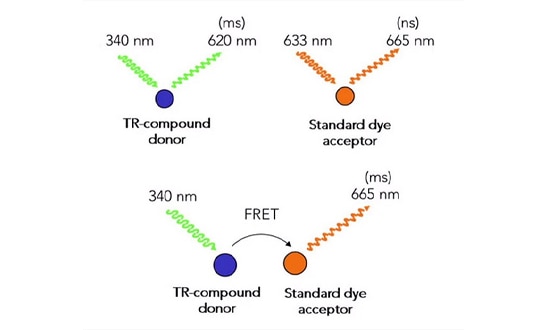
Fluorescence (Förster) resonance energy transfer (FRET) is a physical phenomenon first described over 70 years ago 1. FRET relies on the distance-dependent transfer of energy from a donor molecule to an acceptor molecule. FRET is the radiationless transmission of energy from a donor molecule to an acceptor molecule. The donor molecule is the dye or chromophore that initially absorbs the energy, and the acceptor is the chromophore to which the energy is subsequently transferred. The transfer of energy leads to a reduction in the donor’s fluorescence intensity and excited state lifetime, and an increase in the acceptor’s emission intensity. A pair of molecules that interact in such a manner that FRET occurs are often referred to as donor/acceptor pairs. When the donor molecule has an exceptionally long emission lifetime, such as with the lanthanides, a delay between the sensation of excitatory light and the measurement of FRET emission can be used to decrease background signal. This process is referred to as TR-FRET (Figure 1).
Several different homogeneous proximity assays have been developed. For example, the scintillation proximity assay (SPA) depends on the close-proximity of a radio-labeled molecule to a bead containing a scintillant for signal 2. Bioluminescence resonance energy transfer (BRET) requires that a bioluminescent moiety to be close enough to a green fluorescent protein molecule to allow the transfer of energy 3. Time resolved fluorescence resonance energy transfer (TR-FRET) and HTRF® require that two fluorescent molecules be in close proximity, with the donor fluor having an exceptionally long decay half-life 4-5. Amplified Luminescent Proximity Homogeneous Assay (ALPHA) Screen assays use donor beads that generate singlet oxygen that will interact with acceptor beads located within 200 nm to generate a chemiluminescent signal 6. Spatial proximity analyte reagent capture luminescence (SPARCL) assays use the proximity of a chemiluminescent substrate (acridine) and an oxidative enzyme (HRP) to produce a flash of light proportional to the amount of analyte when the trigger compound H2O2 is added 7.
Below you’ll find useful resources for conducting a range of proximity assays, including featured application descriptions and tips for assay optimization.
Featured Applications

Receptor-Mediated Stimulation of AKT pan-kinase in NIH3T3 Cells
Cell proliferation is usually initiated though cell surface receptors, which interact with specific ligands. This interaction elicits a signal cascade that transmits from the cell surface to the nucleus. The signal cascade involves the activation of proteins by phosphorylation as a result of specific protein kinases. AKT pan is a key protein in this signaling pathway that is activated by phosphorylation. Thus, the ability to monitor the phosphorylation status of this protein can provide insight to the growth status of cells in culture.

A Homogeneous FRET-Based HTS Assay for Quantification of pRb in Cancer Cell Lines to Monitor Inhibition of G0 /G1 Cell Phase Transition
The field of cancer biology remains one of the most rapidly expanding areas of investigation in both industry and academia. Understanding disruptions that control pathways and checkpoints leading to uncontrolled tumor progression are key to understanding disease progression. Presented here is a novel HTRF cell-based assay that quantifies endogenous phosphorylated retinoblastoma protein as a readout of the G0 /G1 cell phase transition.
eBook: Advancing Proteomic Research with Proximity Assays
There are many ways to investigate protein interactions in cells, but various limitations make monitoring these interactions difficult. Combining novel assay technology, software, and instrumentation makes high-throughput detection possible for multiple applications.
In this eBook, you will learn:
- How to monitor the phosphorylation status of AKT pan
- The use of a novel HTRF cell-based assay to quantify endogenous phosphorylation retinoblastoma protein
- A new tool for investigating protein-protein interactions in living cells
- Enabling high-throughput detection of phosphorylation events by combining a novel assay and instrumentation
Product Spotlight
Agilent BioTek Synergy Neo2 Hybrid Multimode Reader
BioTek Synergy Neo2 is an ideal platform to measure a variety of proximity assays. In addition to the xenon flash lamp, the Synergy Neo2 can be configured with a 337 nm nitrogen laser for TR-FRET reactions such as the HTRF® or THUNDER™ assays. Likewise, AlphaScreen™ assays can be enabled with an optional 100 mW, 680 nm diode laser. The multiple light sources along with multiple PMTs allow for simultaneous capture two separate emissions. This fiber-less design provides direct illumination for very strong sample excitation which guarantees the highest levels of sensitivity. The optical system uses barcoded filter cubes containing dichroic mirrors and deep blocking bandpass filters for error free determination of excitation and emission. The reader is controlled, and the data reduction performed by Agilent BioTek Gen5 software. This combination of assay technology, software and instrumentation provides an ideal solution for high-throughput detection.
Tek Tips
Optimizing microplate reader settings
Proximity assays use energy transfer from a donor to an acceptor moiety. FRET based assays employ two fluorescent molecules with overlapping emission and excitation wavelengths in a manner such that the excitation of the donor will result in emission from the donor molecule and the acceptor. While the FRET signal obtained from the acceptor molecule’s emission is generally considered the more important result, information can also be gleaned from the donor molecule’s emission signal. The dual PMT configuration allows for the simultaneous capture of both emission signals, however it is important that both are in the dynamic range of the PMTs. In general, the donor molecule emission will be significantly greater than that observed from the FRET reaction so the gain setting of the two PMTs needs to be set differently to reflect this. While the gain setting for each can be set manually, the AutoScale option in the Gen5 software will automatically integrate the plate to identify the highest well and set the gain appropriately. The Synergy Neo2 also offers an extended dynamic range that extended the signal range 100-fold.
Maximizing the assay window
The dual signal output of FRET based assays can also be used to correct for well-to-well pipetting variances, as well as increase the assay window. Rather than plotting the change in FRET signal against agonist compound concentration directly, plotting the ratio of the FRET signal to that of the donor molecule fluorescence against drug concentration can often have several advantages. The ratiometic nature of the determination often corrects for pipetting errors in assay reagents. With large FRET signals changes both the acceptor emission and donor emission will change significantly in opposite directions with drug concentration. This bifold change will dramatically increase the assay window in terms of fold-change.
Webinar
Expression and Intracellular Translocation of Cancer Biomarkers in Hepatocarcinoma Cells Induced by Changes in Mitochondrial Metabolism
Wednesday, March 22, 2023 | 12PM EST
Presenter: Monika Gooz, M.D. Ph.D., Associate Professor of Drug Discovery & Biomedical Sciences, CZI Imaging Scientist, Silicon Valley Community Foundation, Medical University of South Carolina
Hepatocarcinoma is one of the most common cancer types in the U.S. with limited treatment options and poor survival rate. Recently, cancer bioenergetics emerged as a promising source of targets to develop novel anticancer agents. The identification of relevant biomarkers is essential for screening and validation of small molecules proposed as chemotherapeutic agents. In this regard, there is a great need for high-throughput/high-content methods, which require short, reproducible sample preparation techniques. This application note utilizes the erastin-like compound X1, which is known to modulate cancer cell bioenergetics, and the well-characterized SNU-449 liver cancer cell line as the model system. As readout, directly and indirectly labeled fluorescent antibodies were employed to visualize and quantify expression of proliferative and antiproliferative markers Ki-67 and cytoskeleton-associated protein 4/p63 (CKAP4/p63), as well as the tumor suppressor protein p53. Using the Agilent BioTek Cytation 5 cell imaging multimode reader and Agilent BioTek Gen5 microplate reader and imager software, these markers and the corresponding antibodies were successfully validated for studying the long-term effect of mitochondrial bioenergetics modulators on cancer cells. This method allows a fast and reliable screening of potential drug candidates for further development.
Virtual Summit
Augmented Microscopy Virtual Summit
April 26th - 27th, 2023
For centuries light microscopy has served as an essential instrument of discovery across the life sciences. Ten years ago, Agilent BioTek built upon this foundation with the introduction of the Cytation and the concept of augmented microscopy—creating an automated solution to take researchers from image capture to publication-ready data. Since its launch, the Cytation line of instruments has continued to evolve, while researchers around the world have harnessed its capabilities to push the frontiers of scientific knowledge. We are proud to highlight and celebrate a sampling of these compelling research stories with the 2023 Agilent BioTek Augmented Microscopy Virtual Summit. This event will provide a unique opportunity to learn about diverse imaging-based applications presented by an international panel of researchers and Agilent scientists. The online conference format enables individuals to attend talks and engage live with each presenter.
The two-day event will include:
- More than 20 presentations from researchers across academic, biotech, CRO, and pharmaceutical institutions within North America, Europe, Asia, and Australia. Presentation topics include oncology, neuroscience, aging, virology, cellular metabolism, agriculture, among others
- Agilent BioTek technology presentations covering imaging-based applications, workflows, assay optimizations, and tip and tricks
- Training videos describing Agilent BioTek imaging instrumentation, software, and automation solutions
- Opportunities to discuss your laboratory’s applications with Agilent BioTek Field Application Scientists
Promotion
4-Plate Reader for a 3-Plate Price!
25% off the Agilent BioTek LogPhase 600 multiplate absorbance reader
The Agilent BioTek LogPhase 600 microbiology reader is in a class of its own, designed for measuring microbial growth curves in up to four standard 96-well microplates at a time.
References
- Förster T (1948). Zwischenmolekulare Energiewanderung und Fluoreszenz [Intermolecular energy migration and fluorescence]. Annalen der Physik (in German). 437 (1–2): 55–75. Bibcode:1948 AnP...437...55F. doi:10.1002/andp.19484370105
- Park, Y-W, R.T. Cummings, L. Wu, S. Zheng, P.M. Cameron, A. Woods, D.M. Zaller, A.I. Marcy, and J.D. Hermes (1999) Homogeneous Proximity Tyrosine Kinase Assays: Scintillation Proximity Assay versus Homogeneous Time-Resolved Fluorescence. Analytical Biochemistry 269: 94-104.
- Ward, W. W., Cormier, M. J., (1979). An energy transfer protein in coelenterate bioluminescence. Characterization of the Renilla green-fluorescent protein. J. Biol. Chem, 254:781-788.
- Degorce, F., A. Card, S. Soh, E. Trinquet, G. P. Knapik, and B. Xie (2009) HTRF: A Technology Tailored for Drug Discovery- A Review of Theoretical Aspects and Recent Applications, Curr. Chem. Genomics, 3:22-32
- Comley J. (2006) TR-FRET Based Assays- getting better with age Drug Discovery World Spring pp.22-37.
- Eglen, R.M., T. Reisine, P. Roby, N. Rouleau, C. Illy, R. Bossé, and M. Bielefeld (2008) The Use of AlphaScreen Technology in HTS: Current Status, Curr. Chem. Genomics, 1:2-10.
- Akhavan-Tafti, H. Binger, D.G. et al. (2013) A homogeneous chemiluminescent immunoassay method, J Am. Chem. Soc., 135: 4191-4194.