The PD-L1 IHC 28-8 pharmDx interpretation training is a comprehensive program with in-depth content and practice cases focused on helping pathologists to:
- Understand the role of immune checkpoint pathways
- Learn about technical considerations for optimal slide staining results
- Learn how to evaluate non-squamous NSCLC or melanoma specimens stained with PD-L1 IHC 28-8 pharmDx
Enhanced non-squamous NSCLC and melanoma training programs with virtual microscope for increased confidence in scoring PD-L1 stained slides.

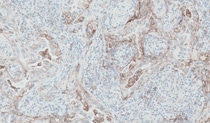
Non-squamous NSCLC Training
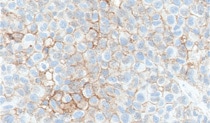
Melanoma Training
Intended Use
For in vitro diagnostic use
PD-L1 IHC 28-8 pharmDx is a qualitative immunohistochemical assay using Monoclonal Rabbit Anti-PD-L1, Clone 28-8 intended for use in the detection of PD-L1 protein in formalin-fixed paraffin-embedded (FFPE) non-squamous non small cell lung cancer (NSCLC) and melanoma tissue using EnVision FLEX visualization system on Autostainer Link 48. PD-L1 protein expression is defined as the percentage of tumor cells exhibiting positive membrane staining at any intensity.
PD-L1 expression as detected by PD-L1 IHC 28-8 pharmDx in non-squamous NSCLC may be associated with enhanced survival from OPDIVO® (nivolumab) treatment.
PD-L1 expression as detected by PD-L1 IHC 28-8 pharmDx in melanoma may be used as an aid in the assessment of patients for whom OPDIVO® (nivolumab) and YERVOY® (ipilimumab) combination treatment is being considered.