
- KEYNOTE-024 and KEYNOTE-010 NSCLC clinical studies determined PD-L1 expression in patients using PD-L1 IHC 22C3 pharmDx.
- Assessment of PD-L1 expression demonstrated staining across the dynamic range of 0-100% positive tumor cells and 0-3+ staining intensity
- PD-L1 IHC 22C3 pharmDx includes the proprietary Mouse Monoclonal Anti-PD-L1, Clone 22C3
- Clone 22C3 does not cross-react with human PD-L2 (Programmed Death Ligand 2) protein
- Clone 22C3 binds to PD-L1 on the cell membrane of tumor cells, immune cells, and cells of epithelial origin
In the KEYTRUDA KEYNOTE-024 study, patients were tested using PD-L1 IHC 22C3 pharmDx. Only patients with TPS ≥ 50% were included in the study.
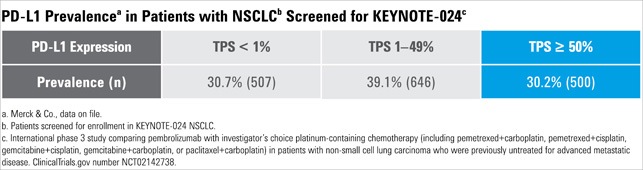
In the KEYTRUDA KEYNOTE-010 study, patients were tested using PD-L1 IHC 22C3 pharmDx. Only patients with TPS ≥ 1% were included in the study.

1. PD-L1 IHC 22C3 pharmDx - Package Insert
2. KEYTRUDA is a registered trademark of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. See the KEYTRUDA product label for expression cutoff values guiding therapy in specific clinical circumstances.
3. Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387(10027):1540-50.
4. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. NEJM 2016. DOI: 10.1056/NEJMoa1606774.
Send us an email and we’ll get back to you
For in vitro diagnostic use
PD-L1 IHC 22C3 pharmDx is a qualitative immunohistochemical assay using Monoclonal Mouse Anti-PD-L1, Clone 22C3 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC) tissue using EnVision FLEX visualization system on Autostainer Link 48. PD-L1 protein expression is determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity. The specimen should be considered to have PD-L1 expression if TPS ≥ 1% and high PD-L1 expression if TPS ≥ 50%.
PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying NSCLC patients for treatment with KEYTRUDA® (pembrolizumab). See the KEYTRUDA® product label for expression cutoff values guiding therapy in specific clinical circumstances.