Revised USP 1058 - Are You Compliant?
Revised USP <1058>
Are You Compliant?

Minimize your audit and regulatory inspection risk while complying with the latest USP <1058>
Instruments within regulated laboratories are required to be qualified periodically with adherence to the 2017 USP <1058> Analytical Instrument Qualification (AIQ) guidelines.
Pharmaceutical labs—or any lab producing results subject to regulatory requirements—must demonstrate and document the suitability of analytical instruments for their intended use. Labs can address these requirements by performing an analytical instrument qualification.
The United States Pharmacopeia is the only major Pharmacopeia with a general chapter dedicated to AIQ: USP <1058>. Therefore, changes to <1058> are of global importance. The 2017 version of USP <1058> is a key regulatory document with significant implications for your laboratory.
Agilent takes an integrated, life cycle-based approach to AIQ. We can help you implement a cost-effective qualification process and align your SOPs to comply with the 2017 USP <1058> requirements.
What you need to know about the 2017 USP <1058>
Instrument qualification—life-cycle approach
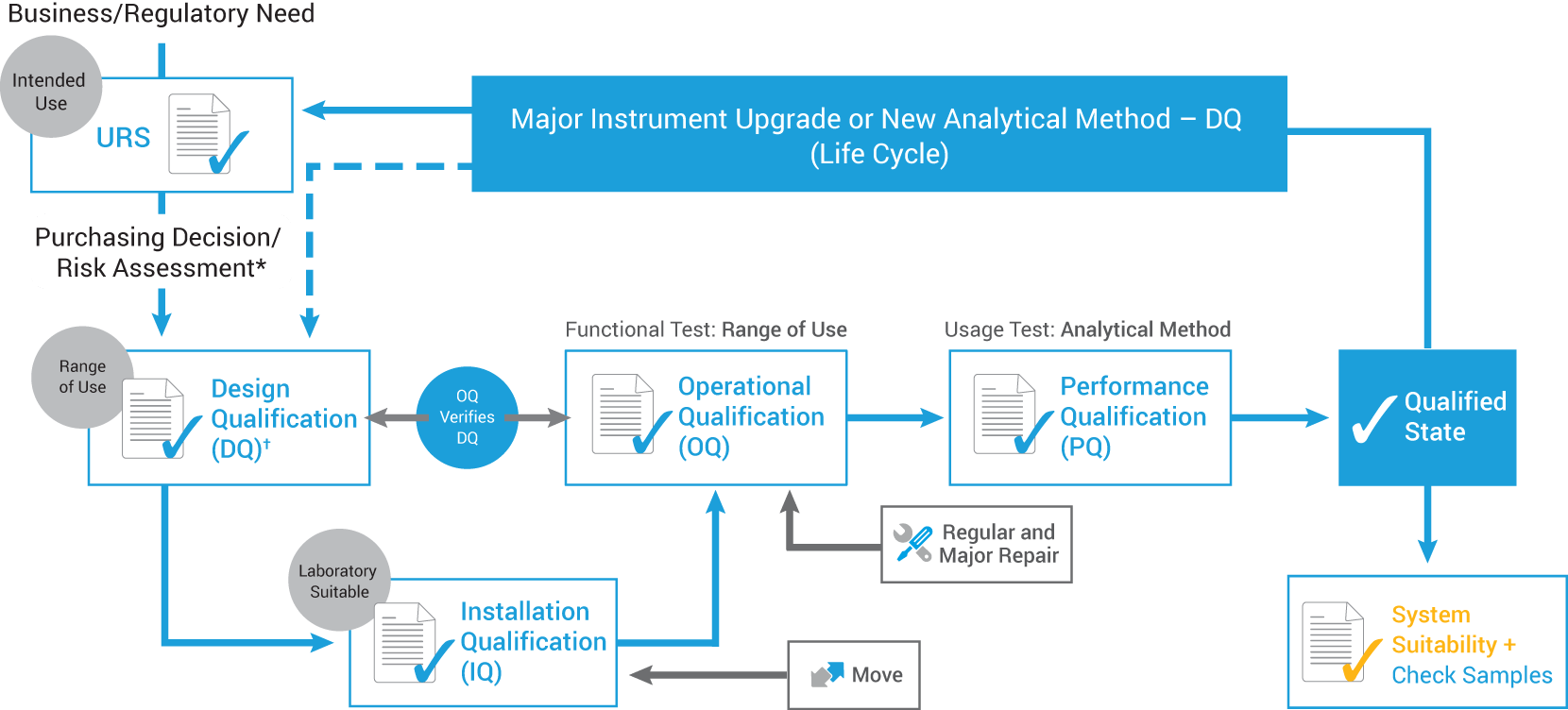
*Required by <1058> to identify instrument categorization (i.e., A, B, or C) and testing requirements.
†The detailed relationship between the URS and DQ depends on the life-cycle process followed. For practical purposes, it is convenient to specify the range of use in the DQ in this model.
Instrument qualification life cycle
The revised 2017 USP <1058> reflects how AIQ has evolved to incorporate the entire instrument life cycle. Some of the guidelines have been retained, including:
- Instrument Groups A, B, and C (although examples have been removed)
- The “4Qs”: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)
However, there are many key differences that clarify the roles of the 4Qs, as well as their relationships to each other. In addition, the software section has been considerably expanded.
The most critical changes include:
- URS (User Requirement Specification) is now required.
- OQ testing is now “tied” to URS/DQ.
- OQ (functionality and range of use) is now required.
- PQ (intended use/application) is now required.
- DQ, OQ, and PQ are now dynamically aligned to instrument use.
Qualification continuum

*The need to include a specific Requirements Trace Matrix in the qualification life cycle is dependent on the intended use, instrument complexity, and company policy. GAMP is a registered trademark of ISPE. See ISPE website for GAMP publications: www.ispe.org/publications/guidance-documents
Integrated approach to hardware qualification and software validation
Significant expansion of software requirements—combined with the removal of instrument examples for groups A, B, and C—means that USP <1058> is now more closely aligned with GAMP 5.
Historically, USP <1058> has simplified the process of introducing group A and B instruments into regulated laboratories. The 2017 update extends this benefit to some group C instruments.
However, the 2017 USP <1058> requirements specify a risk assessment to identify a category for each instrument. Therefore, labs will have to justify their instrument categorizations, and update their procedures to consistently define categorization based on instrument use.
Together, these changes mean that the 2017 USP <1058> can now be considered a continuum of qualification options aligned with the GAMP Good Practice Guide (GPG) for Laboratory Computerized Systems (ISBN 978-1-936379-49-1) and GAMP 5 (ISBN 1-931879-61-3). Specifically:
- Use USP <1058> for standard instruments (for example, HPLC, GC).
- Use GAMP GPG for complex instruments (for example, Q-TOF).
- Use GAMP 5 for Bespoke software validation.
Qualification and data integrity
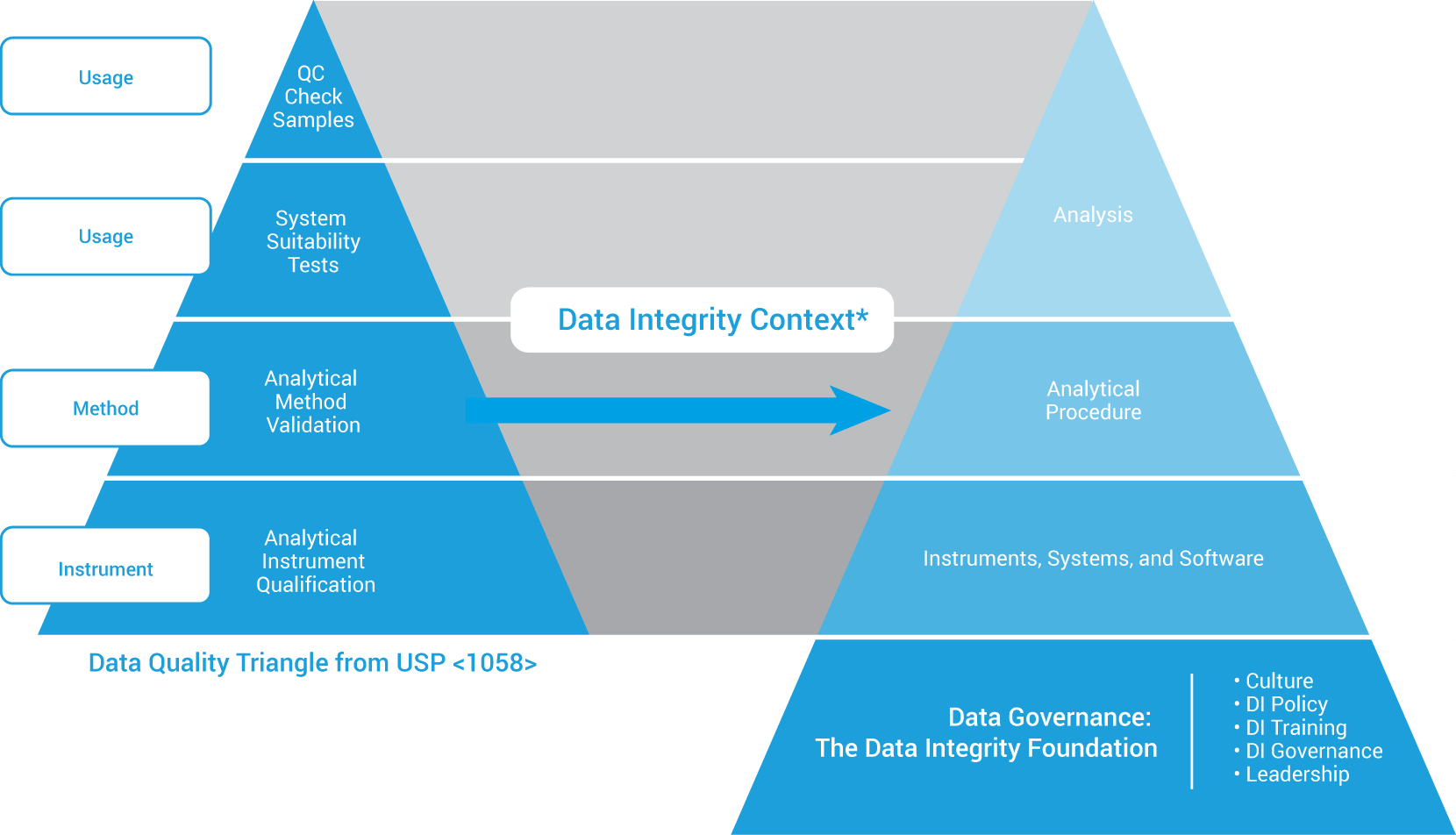
*Adapted with permission from ISBN 978-1-84973-662-6.
The significance of data integrity
Regulatory audit findings associated with data breaches are prompting regulatory agencies worldwide to look more closely at data integrity. For a time, this meant that less attention was paid to traditional audit focus areas, such as AIQ. However, recent FDA 483s indicate that regulators have started looking at the data integrity, traceability, and technical content of AIQ reports in detail once again.
The data quality triangle remains unchanged in the 2017 USP <1058>. However, from a data integrity perspective, the triangle has evolved into the model shown here. In particular:
- AIQ is the foundation of USP <1058> Data Quality Triangle.
- Repeat work or rerun must be managed and explained (documented).
- Data traceability is now a key requirement.
- Data governance is now the foundation of all quality data.