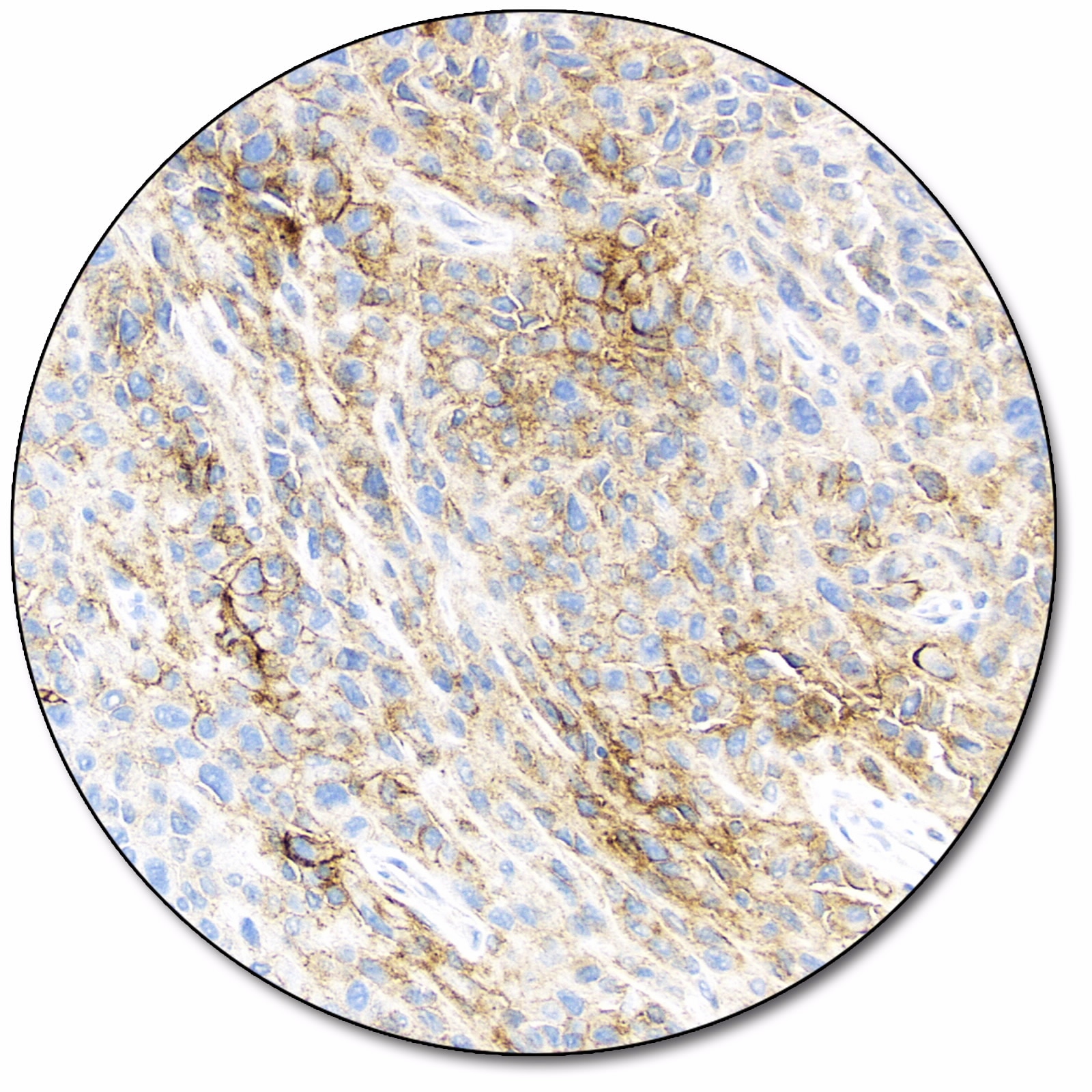
Melanoma
PD-L1 IHC 28-8 pharmDx for Autostainer Link 48
IVD

PD-L1 protein expression in NSCLC, non-squamous non-small cell lung cancer (nsNSCLC), SCCHN, UC, muscle invasive carcinoma (MIUC), melanoma, and esophageal squamous cell carcinoma (ESCC) is determined by using % tumor cell expression, which is the percentage of evaluable tumor cells exhibiting partial or complete membrane staining at any intensity.
PD-L1 protein expression in gastric adenocarcinoma, GEJ adenocarcinoma, and esophageal adenocarcinoma is determined by using Combined Positive Score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100.
Non-small cell lung cancer (NSCLC)
PD-L1 expression (≥ 1% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx is indicated as an aid in identifying early stage NSCLC patients for treatment with OPDIVO® (nivolumab) in combination with platinum-doublet chemotherapy as a companion diagnostic test.
Melanoma (OPDUALAGTM)
PD-L1 expression (< 1% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx is indicated as an aid in identifying melanoma patients for treatment with Opdualag™ (nivolumab and relatlimab) as a companion diagnostic test.
Esophageal squamous cell carcinoma (ESCC)
PD-L1 expression (≥ 1% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx is indicated as an aid in identifying ESCC patients for treatment with OPDIVO® (nivolumab) in combination with fluoropyrimidine and platinum-based chemotherapy or OPDIVO® (nivolumab) in combination with YERVOY® (ipilimumab) as a companion diagnostic test.
Gastric, GEJ, and esophageal adenocarcinoma
PD-L1 expression (CPS ≥ 5) as detected by PD-L1 IHC 28-8 pharmDx is indicated as an aid in identifying gastric, gastroesophageal junction, or esophageal adenocarcinoma patients for treatment with OPDIVO® (nivolumab) in combination with fluoropyrimidine and platinum-based chemotherapy as a companion diagnostic test.
Non-squamous NSCLC (nsNSCLC)
PD-L1 expression (≥ 1% or ≥ 5% or ≥ 10% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx in non-squamous NSCLC (nsNSCLC) may be associated with enhanced survival from OPDIVO® (nivolumab).
Melanoma (OPDIVO® and YERVOY®)
PD-L1 expression (≥ 1% or ≥ 5% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx in melanoma may be used as an aid in the assessment of patients for whom OPDIVO® (nivolumab) and YERVOY® (ipilimumab) combination treatment is being considered.
SCCHN
PD-L1 expression (≥ 1% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx in SCCHN may be associated with enhanced survival from OPDIVO® (nivolumab).
Urothelial carcinoma (UC)
PD-L1 expression (≥ 1% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx in urothelial carcinoma may be associated with enhanced response rate from OPDIVO® (nivolumab).
Muscle invasive urothelial cancer (MIUC)
PD-L1 expression (≥ 1% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx is indicated as an aid in identifying MIUC patients for treatment with OPDIVO® (nivolumab) as a companion diagnostic test
PD-L1 IHC 28-8 pharmDx is subject to an exclusive trademark license to Agilent Technologies, Inc. OPDIVO®, YERVOY® and OPDUALAGTM are registered trademarks owned by Bristol-Myers Squibb.
For more details about PD-L1 IHC 28-8 pharmDx., refer to eIFU.
Reg. Status: For In Vitro Diagnostic Use
For In Vitro Diagnostic Use.