Non-Small Cell Lung Cancer (NSCLC)
PD-L1 IHC 28-8 pharmDx for Autostainer Link 48
IVD

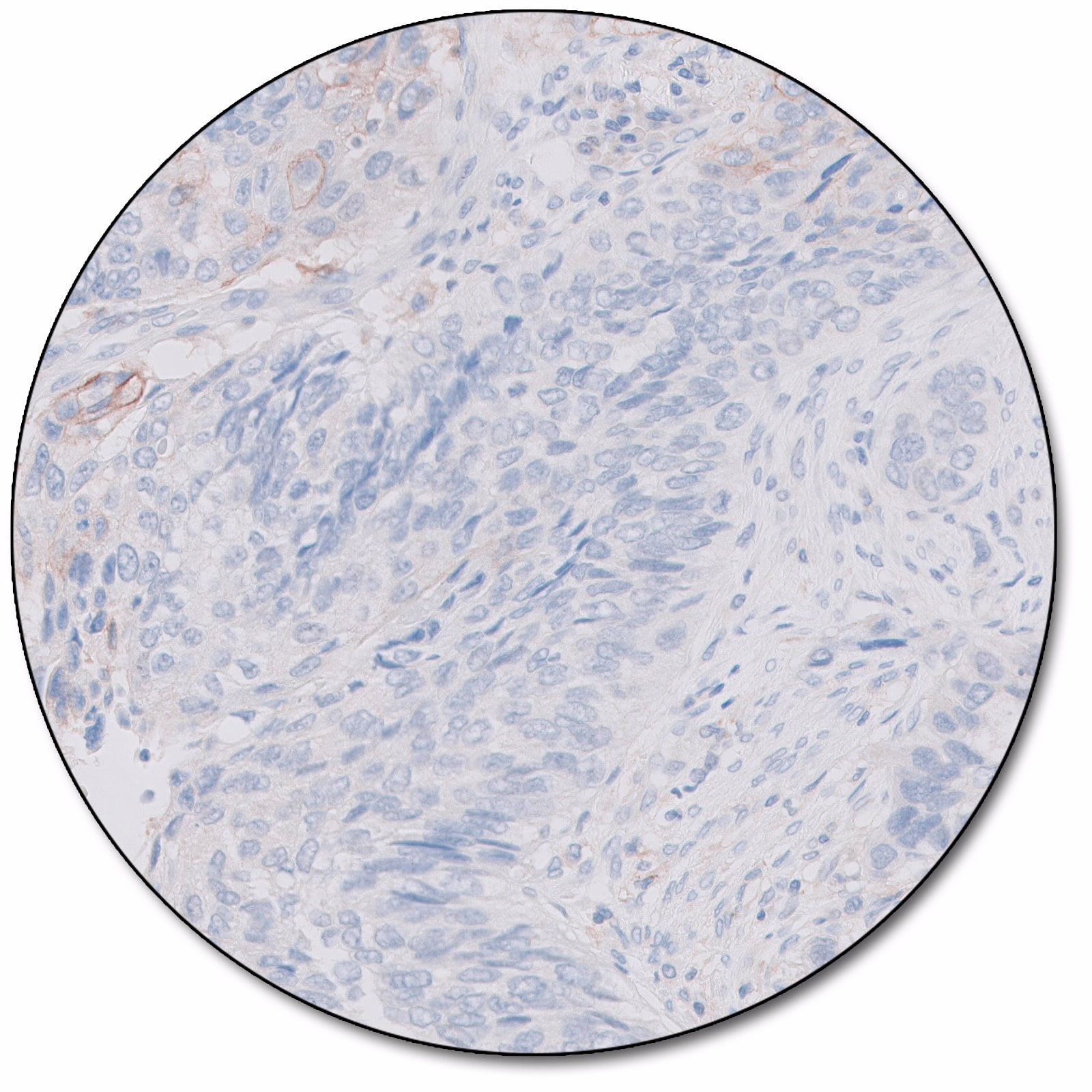
PD-L1 IHC 28-8 pharmDx is a qualitative immunohistochemical assay using Monoclonal Rabbit Anti-PD-L1, Clone 28-8 intended for use in the detection of PD-L1 protein in formalin-fixed, paraffin-embedded (FFPE) non-small cell lung cancer (NSCLC), squamous cell carcinoma of the head and neck (SCCHN), and urothelial carcinoma (UC) tissues using EnVision FLEX visualization system on Autostainer Link 48. PD-L1 protein expression is defined as the percentage of evaluable tumor cells exhibiting partial or complete membrane staining at any intensity.
Tumor indications*
NSCLC – Companion Diagnostic Indication: PD-L1 IHC 28-8 pharmDx is indicated as an aid in identifying NSCLC patients for treatment with OPDIVO® (nivolumab) in combination with YERVOY® (ipilimumab). PD-L1 expression clinical cuttoff ≥ 1% tumor cell expression.
Non-squamous NSCLC: PD-L1 expression (≥ 1% or ≥ 5% or ≥ 10% tumor cell expression), as detected by PD-L1 IHC 28-8 pharmDx in non-squamous NSCLC may be associated with enhanced survival from OPDIVO® (nivolumab).
SCCHN: PD-L1 expression (≥ 1% tumor cell expression), as detected by PD-L1 IHC 28-8 pharmDx in SCCHN may be associated with enhanced survival from OPDIVO® (nivolumab).
UC: PD-L1 expression (≥ 1% tumor cell expression) as detected by PD-L1 IHC 28-8 pharmDx in UC may be associated with enhanced response rate and enhanced disease-free survival from OPDIVO® (nivolumab).
See the OPDIVO® and YERYOV® product labels for specific clinical circumstances guiding PD-L1 testing.
PD-L1 IHC 28-8 pharmDx Kit
The kit includes reagents required for the immunohistochemical staining (except wash buffer), control slides representing different expression levels of PD-L1 protein, and detailed instructions. The kit has been tailored especially for use on Autostainer Link 48 instruments. The materials provided are sufficient for 50 tests (50 slides incubated with monoclonal rabbit antibody to PD-L1 and 50 slides incubated with the corresponding Negative Control Reagent, 100 slides in total).
PD-L1 IHC 28-8 pharmDx is subject to an exclusive trademark license to Agilent Technologies, Inc. OPDIVO® is a trademark owned by Bristol-Myers Squibb.
For In Vitro Diagnostic Use.