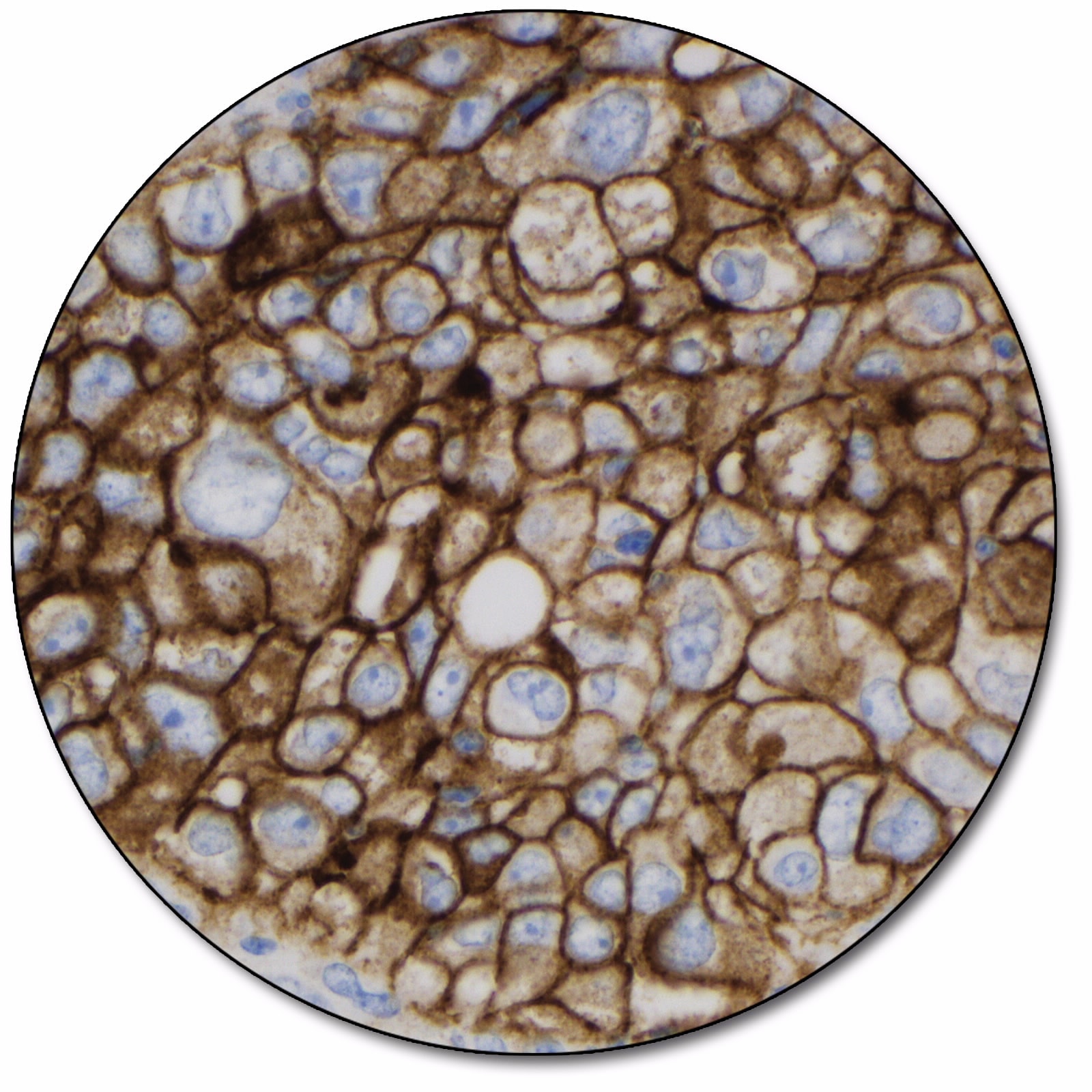
Non-small cell lung cancer
PD-L1 IHC 22C3 pharmDx for Autostainer Link 48
CE-IVD

PD-L1 protein expression in NSCLC is determined by using Tumor Proportion Score (TPS), which is the percentage of viable tumor cells showing partial or complete membrane staining at any intensity.
PD-L1 protein expression in urothelial carcinoma, TNBC, cervical cancer, and gastric or gastroesophageal junction (GEJ) adenocarcinoma is determined by using Combined Positive Score (CPS), which is the number of PD-L1 staining cells (tumor cells, lymphocytes, macrophages) divided by the total number of viable tumor cells, multiplied by 100.
PD-L1 protein expression in HNSCC is determined by using CPS and/or TPS.
PD-L1 protein expression in melanoma is determined by using Melanoma Score (MEL Score), which is the ratio of tumor and associated immune cells expressing PD-L1 at any intensity, relative to all viable tumor cells and PD-L1 staining associated immune cells.
PD-L1 IHC 22C3 pharmDx is indicated as an aid in identifying NSCLC, urothelial carcinoma, HNSCC, TNBC, cervical cancer, and gastric or gastroesophageal junction (GEJ) adenocarcinoma patients for treatment with KEYTRUDA® (pembrolizumab). For indications and PD-L1 expression levels, refer to the full intended use in section 1 of the Instructions for Use (IFU) for PD-L1 IHC 22C3 pharmDx, Code SK006.
For details on staining interpretation, refer to section 13 of the IFU and indication-specific PD-L1 IHC 22C3 pharmDx Interpretation Manuals.
PD-L1 IHC 22C3 pharmDx kit
The kit includes reagents required for the immunohistochemical staining (except EnVision FLEX Wash Buffer and Hematoxylin (Link)), control slides representing positive and negative PD-L1 protein expression and detailed instructions. The kit has been tailored especially for use on Autostainer Link 48 instruments. The materials provided are sufficient for 50 tests (50 slides incubated with monoclonal mouse antibody to PD-L1 and 50 slides incubated with the corresponding negative control reagent, 100 slides in total).
For countries outside of the European Union, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.
KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc.
For In Vitro Diagnostic Use.
