
GMP Production
Process Flow Overview
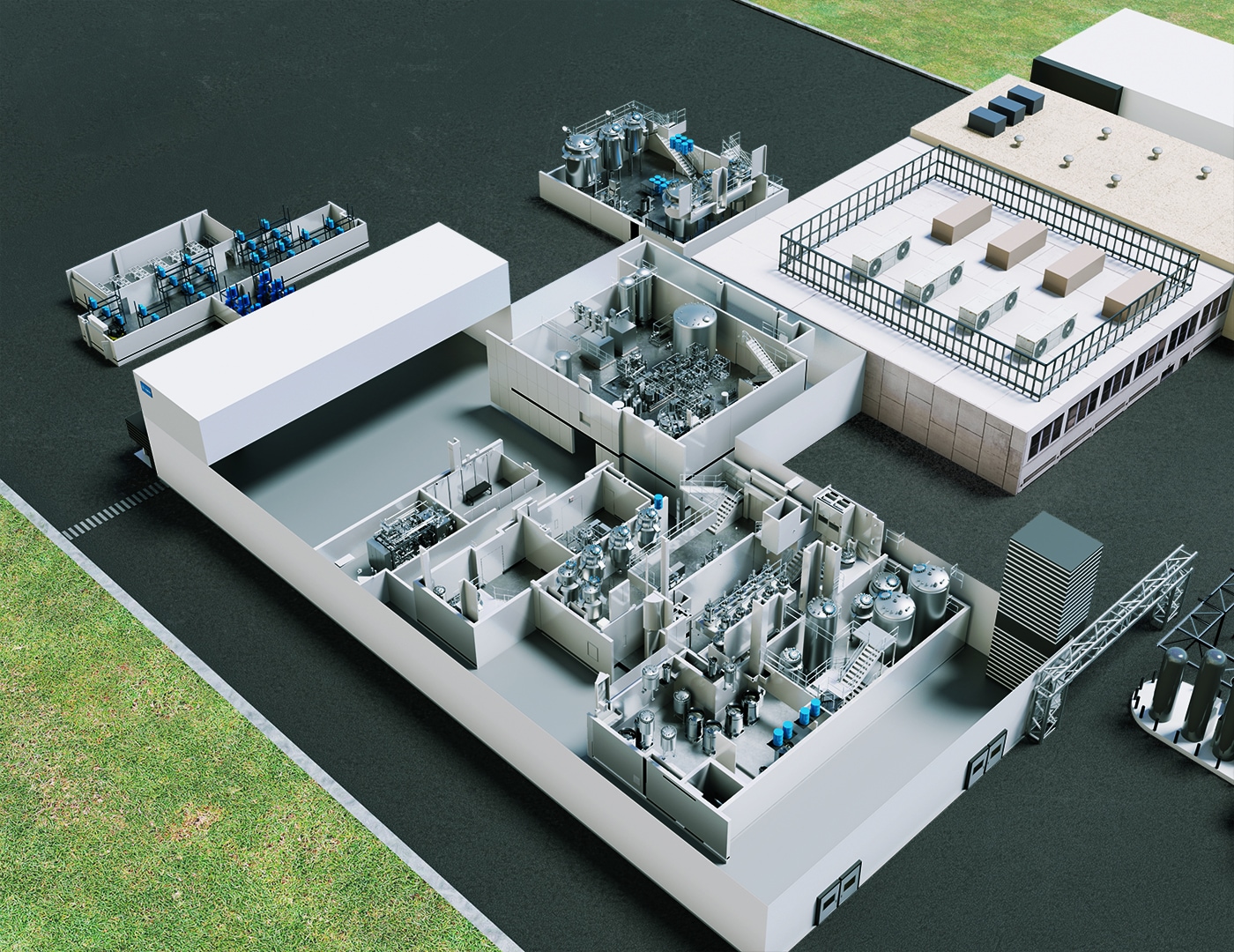
Take a virtual walk through our GMP oligo manufacturing facility and explore the manufacturing processes, including synthesis, purification, and lyophilization, at your own pace. See the manufacturing equipment closer than in real life.

Virtual Tour of Agilent’s Oligonucleotide GMP Manufacturing



Why Choose Agilent for Oligonucleotide Manufacturing?
Agilent, renowned for its laboratory equipment and instruments, is also a key oligonucleotide manufacturer.

Experienced team
Our highly skilled and tenured oligonucleotide chemists drive innovation and quality. Many have been with us since 2006 when our Boulder facility began producing oligo therapeutics.

Regulatory compliance
Our facilities passed FDA pre-approval inspections and received Japanese Pharmaceuticals and Medical Devices Agency (PMDA) accreditation. We assist partners with international regulatory submissions.

Quality
We adhere to cGMP standards, ensuring materials meet rigorous specifications and purity requirements for our partners.

Commercial success
Agilent manufactures six commercially approved oligo therapeutics, available globally—more than any other oligo Contract development and manufacturing organization (CDMO).
To see
our locations and
our portfolio

Name of the instrument
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Praesent maximus in erat sed consectetur. Maecenas sodales pellentesque est, eu pellentesque purus mattis non. Phasellus ullamcorper tincidunt laoreet. Suspendisse et ex scelerisque, lobortis lectus vel, efficitur dolor. Curabitur pharetra pretium diam sed volutpat.
Drag the screen to move around



Reach out to the Agilent Nucleic Acid Solutions Team at pdl-boulderinfo@agilent.com