PD-L1 IHC 22C3 pharmDx, Code GE006
IVD

For in vitro diagnostic use
PD-L1 IHC 22C3 pharmDx, Code GE006, is a qualitative immunohistochemical assay using Monoclonal Mouse Anti-PD-L1, Clone 22C3 intended for use in the detection of PD-L1 protein in certain formalin-fixed, paraffin-embedded (FFPE) cancer tissues using EnVision FLEX visualization system for use on Dako Omnis.
PD-L1 IHC 22C3 pharmDx, Code GE006, is indicated as an aid in identifying certain cancer patients for treatment with KEYTRUDA® (pembrolizumab). See the KEYTRUDA® product label for approved indications and expression cutoff values guiding therapy and specific clinical circumstances guiding PD-L1 testing.
PD-L1 IHC 22C3 pharmDx, Code GE006, is intended for use in specific types of cancer, which may vary by region. Please refer to the local Instructions for Use for a description of the types of cancer in which PD-L1 IHC 22C3 pharmDx, Code GE006, is intended for use. Instrument compatibility may also vary by region. Please refer to the region-specific Instructions For Use to confirm compatibility with your instrument setup.
PD-L1 IHC 22C3 pharmDx, Code GE006, includes 12 mL of PD-L1 Primary Antibody and 12 mL of Negative Control Reagent in ready-to-use format. The assay is a modular IHC assay for 60 tests: 60 slides incubated with the primary antibody to PD-L1 protein and 60 slides incubated with the Negative Control Reagent.
Refer to the region-specific Instructions For Use for the complete list of materials that are required but not supplied with PD-L1 IHC 22C3 pharmDx, Code GE006.
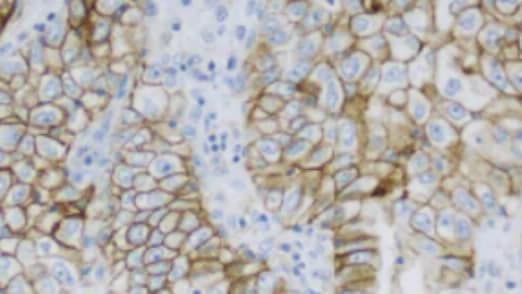
For details on staining interpretation, refer to the region-specific Instructions For Use and indication-specific PD-L1 IHC 22C3 pharmDx Interpretation Manuals.
PD-L1 IHC 22C3 pharmDx, Code GE006, is subject to an exclusive trademark license to Dako Denmark A/S. KEYTRUDA is a registered trademark of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
For In Vitro Diagnostic Use.