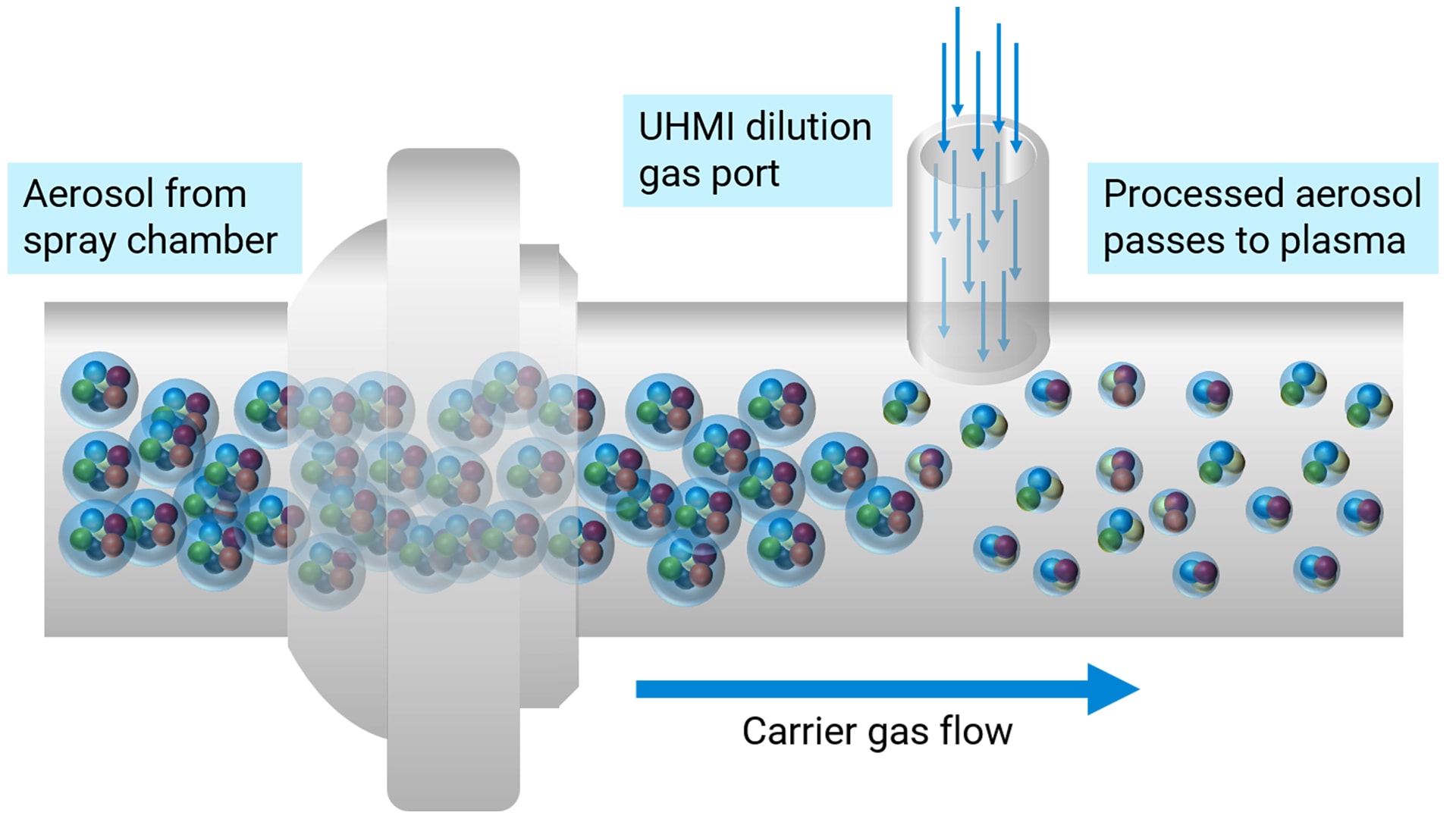
Fundamental design: The Agilent range of ICP-MS and ICP-QQQ instruments share many design features, ensuring that the Agilent ICP-QQQ instruments (non-semiconductor configuration) offer the same matrix tolerance and robustness as Agilent’s market-leading single quadrupole ICP-MS systems. These instruments benefit from a high-energy plasma (indicated by a low 1% CeO/Ce ratio) and an ultra-high-matrix-introduction (UHMI) aerosol dilution technique that extends matrix tolerance to up to 25% total dissolved solids (TDS), making them ideal for routine applications.
Software: Both types of instruments are controlled by Agilent ICP-MS MassHunter software, which includes a host of usability tools. These features aid method development (Method Wizard, IntelliQuant), configuration (preset methods, including EPA methods), optimization (lens autotuning), data acquisition, reporting, and instrument maintenance (EMF), thereby simplifying instrument use.

Collision/reaction cell (CRC) technology: Agilent ICP-MS and ICP-QQQ instruments share the same CRC—the Octopole Reaction System (ORS4)—which can operate in helium (He) collision mode, enhanced high-energy helium mode (HEHe mode), or with a reactive cell gas. Helium mode addresses many of the polyatomic (molecular) interferences that impede accurate analysis of critical trace elements via kinetic energy discrimination (KED) and collisionally-induced dissociation (CID).
He KED separates larger, polyatomic interfering ions from the smaller, more mobile monatomic analyte (the larger interfering ions collide with He atoms more frequently, losing energy) by applying an energy differential at the cell exit. While He KED mode can be used to attenuate most interferences that arise from the plasma gas or sample matrix, there are some instances when HEHe or a reactive cell gas is needed. Examples include trace-level measurement of elements such as silicon (Si), phosphorus (P), and sulfur (S). Also, He KED cannot resolve isobaric (same mass) and doubly charged ion (M++) overlaps on analytes.
Discrete sampling: The Agilent AVS MS accessory can boost the productivity of both ICP-MS and ICP-QQQ, ideal for high-throughput routine applications
Differences between Agilent ICP-MS and ICP-QQQ instruments
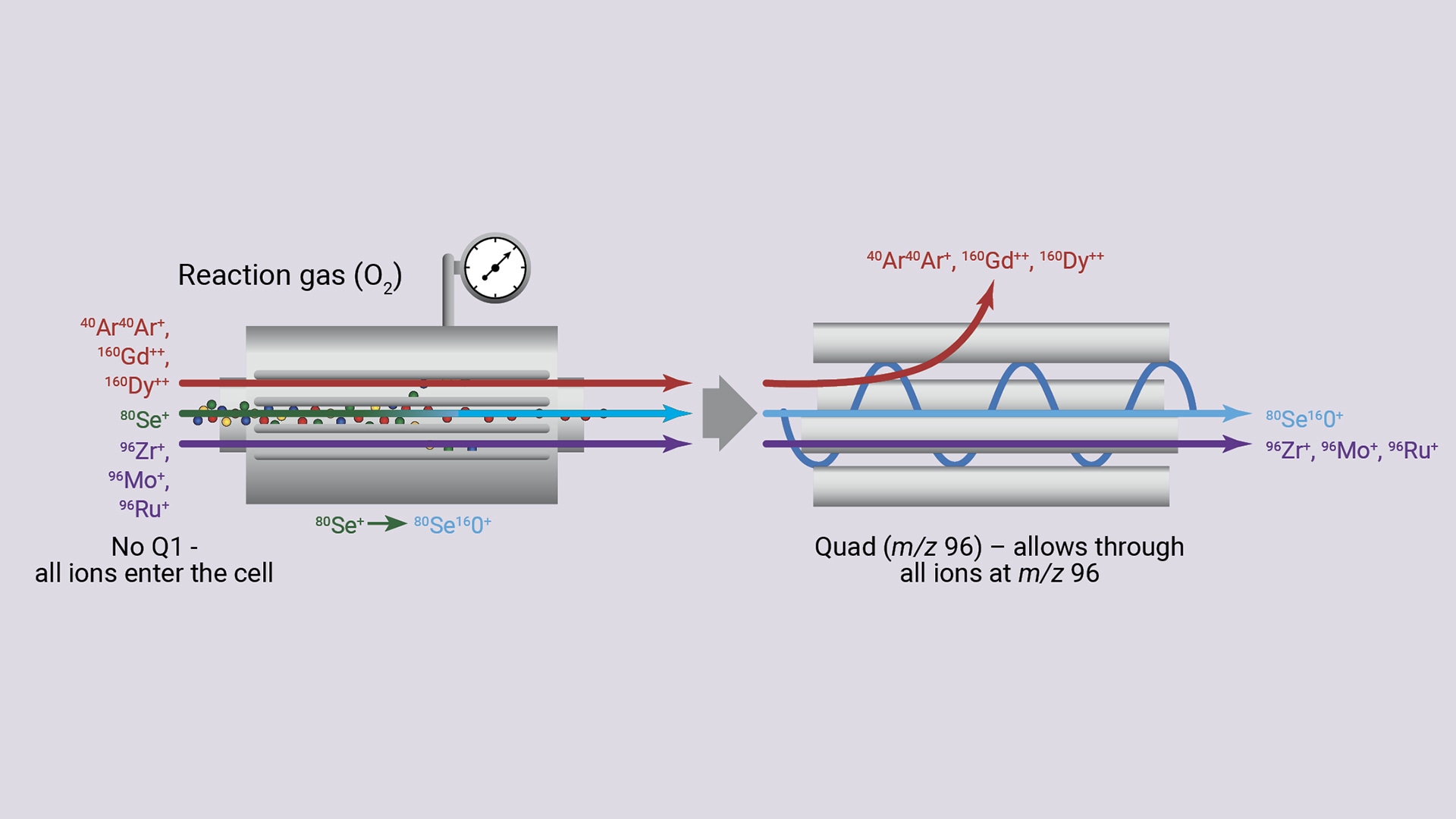
A single-ion mass filter: Agilent SQ ICP-MS instruments are routinely operated with a simple reactive gas, such as hydrogen (H2), to remove interferences for a few selected analytes. However, when a more reactive gas, such as oxygen (O2) or ammonia (NH3), is introduced into the CRC to analyze complex, variable-matrix samples, many ions will react with the cell gas to form reaction product ions.
These unwanted polyatomic ions can interfere with other analytes, creating additional problems rather than resolving them. Alternatively, as shown in Figure 3 for the analysis of selenium (80Se) using O2 as the cell gas, the analyte forms 80Se16O+ in the cell. This product ion, with a mass-to-charge ratio (m/z) of 96, can be measured free of interference from ions with an m/z of 80. However, interfering ions at m/z 96 (Zr, Mo, and Ru) would lead to false-positive results for Se (measured as SeO) using single-quadrupole ICP-MS.
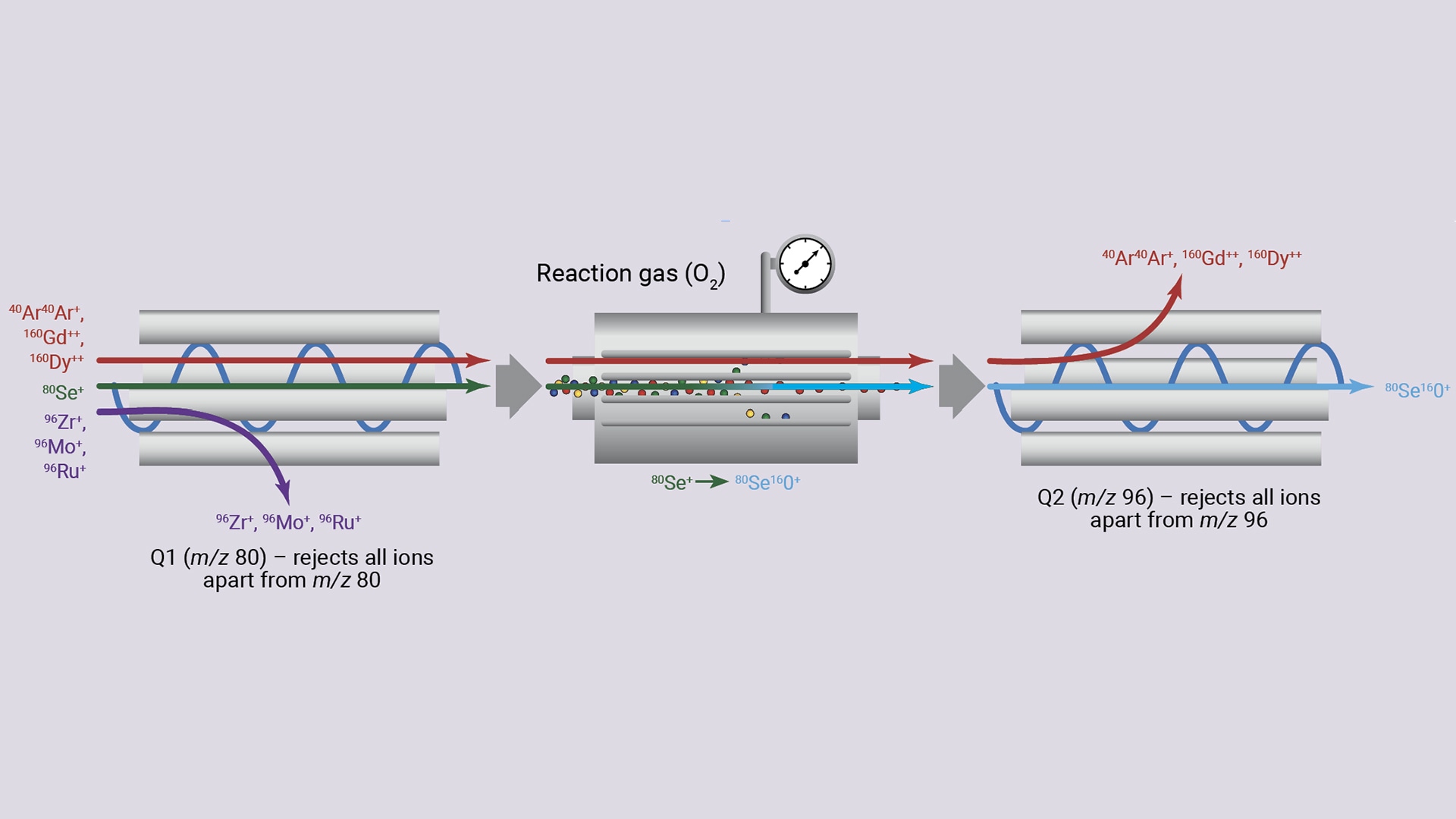
Two ion mass filters: To address this challenge, Agilent ICP-QQQ systems include another unit mass (1 u) quadrupole mass filter, Q1, before the CRC to select which ions enter the cell to react with the reactive cell gas (Figure 4). Any ions that exit the cell are then filtered by the second quadrupole (Q2), before being passed to the detector.
This tandem mass spectrometer operation (ICP-MS/MS) provides precise control of reaction chemistry in the CRC, making the technique useful for routine measurements of varied sample types. The addition of a second quadrupole also improves the abundance sensitivity (AS) of ICP-QQQ, reducing peak tail overlaps.
Routine applications of ICP-QQQ
For most routine sample testing, ICP-QQQ instruments can be operated much like single quadrupole ICP-MS instruments, in He KED mode with Q1 acting as an ion guide rather than a mass filter.
However, for analytes subject to intense interferences, including isobaric or M++ interferences, Q1 ensures that only ions with the same m/z enter the CRC at a time, rejecting all other ions. This MS/MS configuration provides greater control over interferences in the reaction mode, thereby enabling accurate measurements in complex matrices.
Even higher-mass elements that may traditionally be thought of as interference-free, such as Cd and Hg, benefit from ICP-QQQ. Cadmium can be affected by MoO, and mercury by WO and WOH interferences. Many samples contain appreciable levels of molybdenum and even tungsten, which can lead to incorrect analytical results. ICP-QQQ solves these problems using reactive cell gases.
In the next article on this subject, we will highlight application examples of ICP-QQQ in routine testing, so be sure to check out the next issue of The ICP-MS Journal.
