PD-L1 IHC 28-8 pharmDx for Non Small Cell Lung Cancer


CHECKMATE-227 was a Phase III, randomized, multi-center, multi-cohort, open-label study in patients who had no prior anti cancer therapy with metastatic NSCLC. Part 1a of the study investigated patients with PD-L1 expression level < 1%, who were previously untreated for advanced disease. PD-L1 IHC 28-8 pharmDx was the only test used for PD-L1 expression analysis across all histology in non-squamous and squamous NSCLC.
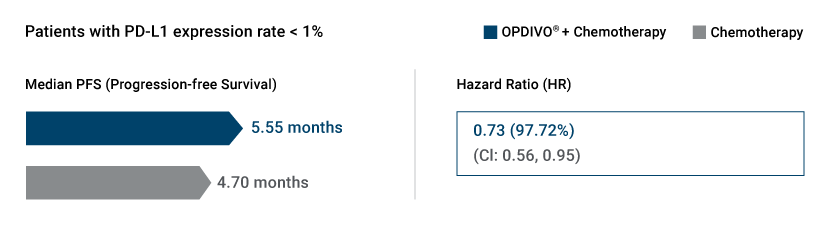
CHECKMATE-227, Part 1b highlighted the median Progression free survival and hazard ratio outcomes from combining OPDIVO® (nivolumab) plus Chemotherapy as a first line treatment for metastatic NSCLC patients whose tumors exhibited PD-L1 expression <1%.
* PD-L1 IHC pharmDx is for in vitro diagnostic use.
PD-L1 IHC 28-8 pharmDx is MHLW-approved and fully validated with analytical performance having met all pre-determined acceptance criteria for sensitivity, specificity and precision.
| Selected analytical validation parameters | Description |
|---|---|
| Specificity |
|
| Sensitivity |
|
| Repeatability |
|
| Reproducibility |
|
OA = Overall Agreement
| Product | Code |
|---|---|
| PD-L1 IHC 28-8 pharmDx | SK005 |
Required but not included in kit: Autostainer Link 48 EnVision FLEX Wash Buffer, 20x EnVision FLEX Hematoxylin (Link) PT Link PT Link Rinse Station |
AS480 K8007 K8008 PT101 / PT200 PT109 |
- CHECKMATE-227. M.D. Hellmann, et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer, The New England Journal of Medicine, 2019.
- PD-L1 IHC 28-8 pharmDx Instructions for Use.
- OPDIVO® package insert. Manufactured by: Bristol-Myers Squibb Company, Princeton, NJ 08543 USA U.S. License No. 1713.
Intended Use
For in vitro diagnostic use.
To measure the proportion of PD-L1 expression in cancer tissue or cells
- Indication as an aid for proper administration of nivolumab [recombinant] in NSCLC patients and head-and-neck cancer patients
- Indication as an aid for proper administration of nivolumab [recombinant] and ipilimumab combination in melanoma patients
It is desirable to measure PD-L1 expression by PD-L1 IHC 28-8 kit in determining whether or not the following drugs can be administered.
- Nivolumab [recombinant] for patients with non-squamous NSCLC or head-and-neck cancer who have been treated with chemotherapy previously
- Combination therapy of nivolumab [recombinant] and chemotherapy for NSCLC patients who have not been treated with chemotherapy
- Combination therapy of nivolumab [recombinant] and ipilimumab for melanoma patients
If it is not possible to measure PD-L1 expression using PD-L1 IHC 28-8pharmDx kit, refer to the package insert of the drugs and appropriately judge the administration's adequacy.




