Access Agilent eNewsletter September 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Agilent Bio-Monolith Protein A and G columns offer more options for mAb titer determination
By Phu T. Duong and Linda L. Lloyd
Agilent Biocolumns
In recent years, monoclonal antibodies (mAbs) have gained importance as one of the dominant biopharma products, in response to growing demand to treat various diseases. These antibodies have been engineered with specific genetic makeup for better targeting of disease agents. During the development of these antibodies, Protein A and G analytical affinity columns are used for determining their titer or concentration from various cell culture supernatants for the selection of the high yield clones.
Protein A and G columns have high affinity for antibodies, and so they bind only to antibodies in cell-culture supernatants. However, they have different selectivity. For example, Agilent Bio-Monolith Protein A columns have high affinity for human subclasses IgG1 and IgG2 and no affinity for IgG3, whereas Agilent Bio-Monolith Protein G columns have high affinity for human subclasses IgG1, IgG2, and IgG3. Conversely, the Protein G column has no affinity for human subclass monoclonal antibodies such as IgA and IgD, but the Protein A column binds to both these antibodies (Table 1). Together, these columns complement each other and enable titer determination of the various mAb subclasses and fragments currently in development as biotherapeutics.
Antibody |
Antibody |
Protein A |
Protein G |
|---|---|---|---|
Human |
Human lgG1 |
++++ |
++++ |
Human lgG2 |
++++ |
++++ |
|
Human lgG3 |
- |
++++ |
|
Human lgG4 |
++++ |
++++ |
|
Human lgA |
++ |
- |
|
Human lgD |
++ |
- |
|
Human lgE |
++ |
- |
|
Human lgM |
++ |
- |
|
Mouse |
Mouse lgG1 |
+ |
++ |
Mouse lgG2a |
++++ |
++++ |
|
Mouse lgG2b |
+++ |
+++ |
|
Mouse lgG3 |
++ |
+++ |
|
Mouse lgM |
+/- |
- |
Antibody Fragments |
Protein A |
Protein G |
|---|---|---|
Human Fab |
+ |
+ |
Human F(ab')2 |
+ |
+ |
Human scFv |
+ |
- |
Human Fc |
++ |
++ |
Human Κ |
- |
- |
Human λ |
- |
- |
Key code for relative affinity of Protein A & G for respective antibodies:
++++ = Strong affinity
+++ = Moderate affinity
++ = Weak affinity
+ = Slight affinity
- = No affinity
Table 1. Binding affinity of Protein A and G to different human and mouse IgG subclasses [1,2]
 Enlarge
Enlarge
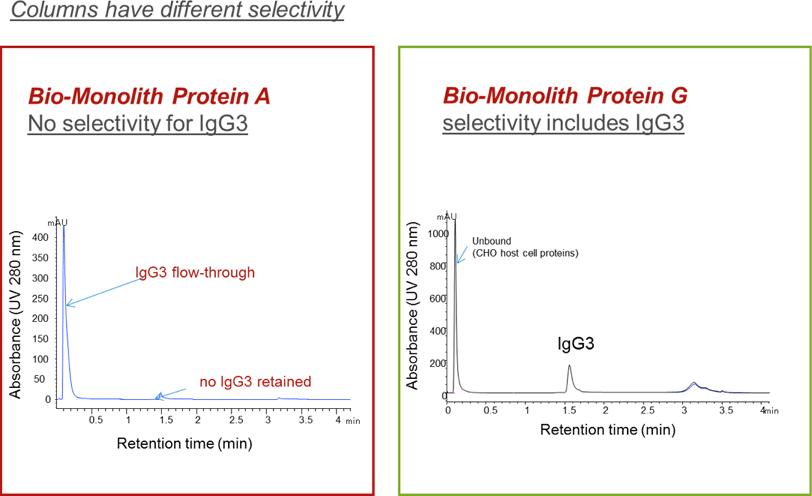
Figure 1. Comparison of selectivity of Agilent Bio-Monolith Protein A and G columns for the capture of IgG3.
Choosing the right column delivers specificity and selectivity for capturing specific mAb in supernatant of cell culture
In the following examples, we look at the complementary characteristics between Agilent Bio-Monolith Protein A columns and Bio-Monolith Protein G columns for human monoclonal antibodies. Figure 1 illustrates the specificity of the columns for mAbs. When the supernatant containing host cell proteins (CHO-cells) was spiked with IgG3 mAbs and injected onto the Agilent Bio-Monolith Protein G column (shown on the right), only the IgG3 was captured. It eluted around 1.6 minutes at 1.0 mL/min, whereas the host cell proteins were not captured by the column and eluted as a flow-through peak. The Agilent Bio-Monolith Protein A column (shown on the left) did not capture the IgG3 sample. As indicated in Table 1, these two columns have different selectivity for antibodies. Figure 1 demonstrates the differences in selectivity between the two columns. Both columns can bind and separate IgG1 and IgG2 (not shown here), but only Bio-Monolith Protein G can capture IgG3 from the supernatant.
 Enlarge
Enlarge
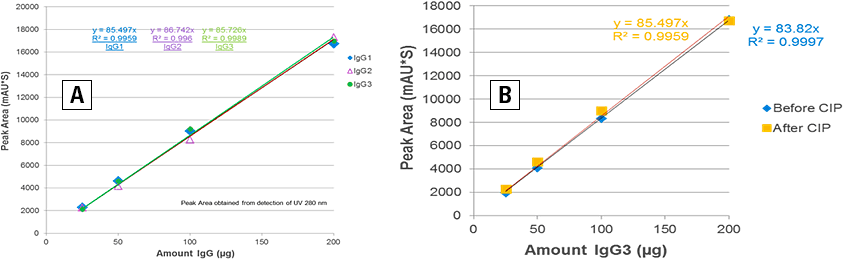
Figure 2. A, peak area linearity; B, before and after clean-in-place, column restores its full capacity and performance.
Accurate quantitation and linearity with Agilent columns
Accurate quantitation of mAb titer of monoclonal antibody is essential during the early stages of development when the cell line is selected and also during manufacture when the amount of mAb in the cell-culture supernatant determines the optimum harvest time. To demonstrate the ability of the Agilent Bio-Monolith Protein A and G columns in the accurate quantitation of mAbs, different amounts (µg) of purified IgGs were injected onto the columns. Data of peak areas vs. amounts of IgGs generated were used to construct the linearity lines to determine the accuracy of the analysis. Figure 2A shows the linearity of peak areas from the Protein G column. The linearity of peak areas showed that the Agilent Bio-Monolith Protein G column can be used for quantitation of mAb in harvest cell-culture media with different concentration ranges. The column was injected as low as 10 µg. The signal to noise (S/N) ratio here was no higher than a 1:1 ratio for the amount of 10 µg (data not shown). Similar data was also obtained from Agilent Bio-Monolith Protein A column (except for IgG3 data) (data not shown here). The maximum loading capacity for both columns is approximately 400 to 500 µg IgG which covers the range of concentrations achieved during cell line selection and production. With their specially designed polymer matrices, Agilent Bio-Monolith Protein A and G columns are very rugged. Their performance is easily restored after clean-in-place (Figure 2B).
Rapid titer determination for a broad range of mAb variants
Agilent Bio-Monolith Protein A and G columns are thus complementary, and so Protein G has affinity for mAbs that do not bind to Protein A and vice versa. These columns provide more options for rapid titer determination for a wider range of mAb variants. With the wide linearity range of the calibration curves titer determination is possible over a wide range of concentrations to achieve accurate quantitation of candidate concentration.
Agilent columns to enhance the speed and accuracy of your biomolecule characterizations
Agilent AdvanceBio columns are designed to enhance the quickness and accuracy of your biomolecule characterization, including monoclonal antibodies (mAbs), other proteins, peptides and synthetic oligonucleotides. Visit our resource page for more information on how the AdvanceBio product family can provide your busy lab with greater efficiencies, higher throughputs, and cost savings.
References
- Richman, D. D., Cleveland, P. H., Oxman, M. N. and Johnson, K. M. 1982. “The binding of 1.
Staphylococci protein A by the sera of different animal species.” J. Immunol. 128: 2300-2305. - Frank, M. B. 1997. “Antibody Binding to Protein A and Protein G beads”. 5. In: Frank, M. B., ed. Molecular Biology Protocols. Oklahoma City.
>> Update My Profile | Subscribe to Access Agilent | Article Directory