Access Agilent eNewsletter October 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory
Reliable pharmaceutical analysis using a Waters Alliance HPLC system with Agilent supplies
By Jignesh Shah, Tiantian Li, and Anil Sharma
Agilent Application Scientists
Acetaminophen, also known as paracetamol, is a common pharmaceutical that is widely used to relieve pain and reduce fever. The preparation and analysis of acetaminophen is clearly defined in the United States Pharmacopeia (USP) [1], and can be conducted on various HPLC systems using columns and sample preparation products from different manufacturers. But what happens when you use an HPLC from one vendor and consumable HPLC parts and supplies from another vendor?
To answer that question, we performed HPLC analysis and method validation for acetaminophen tablets using an Agilent Poroshell 120 column and Waters Alliance HPLC system, equipped with an Agilent CrossLab performance maintenance kit for the pump and autosampler, as well as an Agilent CrossLab deuterium lamp. The results showed excellent linearity, precision, and sample recoveries, indicating that Agilent supplies for Alliance systems provide suitable alternatives to Waters parts.
Hybrid system combined Waters and Agilent products
We performed the HPLC analysis using a Waters Alliance Separation Module (model 2695) with a Waters UV-Vis Detector (model 2487). The system was equipped with an Agilent CrossLab deuterium lamp, and parts from an Agilent CrossLab performance maintenance kit, including plungers, plunger seals, diffuser, face seal, solvent filters, syringe, precolumn filter, check valve cartridges, needle wash frit, needle assembly, and injector seals, among others. Spectral data was obtained with a Waters Acquity photodiode array (PDA) detector.
Parameter |
Value |
|---|---|
|
Column |
Agilent Poroshell 120 EC-C18, 4.6 × 75 mm, 2.7 µm (p/n 697975-902) |
|
Mobile phase |
1:3 Methanol:water, isocratic |
|
Sample dissolving/diluting solution (DDS) |
1:3 Methanol:water |
|
Flow rate |
1.5 mL/min |
|
Column temperature |
25 °C |
|
UV detection |
243 nm |
|
Injection volume |
10 µL |
|
Other Agilent supplies |
|
|
Syringe filters |
Agilent Captiva Premium syringe filter with regenerated cellulose membrane, 0.45 µm pore size (p/n 5190-5111) |
|
Vials |
Amber, write-on spot, 100/pack (p/n 5182-0716) |
|
Vial caps |
Blue, screw cap, 100/pack (p/n 5182-0717) |
Table 1. Conditions for robust analysis of acetaminophen tablets used an Agilent column, lamp, and supplies on a Waters LC.
System qualification tests demonstrated dependable results
After installing the Agilent parts and deuterium lamp in the Alliance HPLC system, we first qualified the system with tests that included pump flow accuracy, wavelength accuracy, signal noise and drift, injection precision, carry-over, response linearity of a caffeine standard, and gradient composition accuracy. All tests passed with excellent results, demonstrating the full functionality of the system.
We then analyzed acetaminophen standards and drug tablet samples following the USP method [1], and performed method validation. While Agilent Application Note 5991-6017EN shows full details of the results, this article provides some interesting highlights.
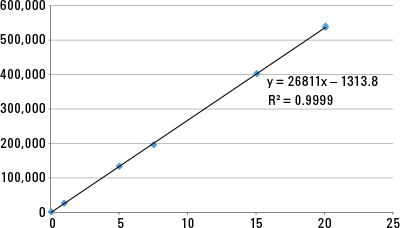
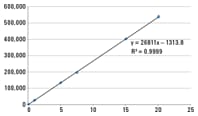 Enlarge
Enlarge
Figure 1. Combined Waters/Agilent system gave linear response of peak area against concentration of acetaminophen.
Linear calibration curve for trouble-free quantitation
The hybrid system gave a linear response to acetaminophen from 0.05 to 20 µg/mL. We analyzed six calibration standards with concentrations of 0.05, 1, 5, 7.5, 15, and 20 µg/mL, and plotted their peak areas against concentration (Figure 1). The regression coefficient R2 was 0.9999, indicating excellent linearity.
Superb accuracy and precision
To demonstrate method accuracy, we analyzed acetaminophen quality control (QC) standards at 0.5 µg/mL and 15 µg/mL and calculated their concentrations using the previously generated calibration curve. To assess method precision, we prepared both QC standards three times independently (three batches), and injected each batch in a separate sequence with six replicates. We assessed the intra-batch precision by the standard deviation between the replicates in the same sequence, and evaluated the inter-batch precision by the variation between different batches. The results of accuracy and precision studies are summarized in Table 2.
QC sample concentration (µg/mL) |
Batch number |
Calculated concentration (µg/mL) |
Accuracy |
Intra-batch RSD (%)
|
Inter-batch RSD (%)
|
|---|---|---|---|---|---|
|
0.5 |
1 |
0.51 |
101.08 |
0.23 |
0.58 |
|
|
2 |
0.51 |
101.41 |
0.42 |
|
|
|
3 |
0.50 |
100.24 |
0.19 |
|
|
15 |
1 |
15.06 |
100.41 |
0.15 |
0.62 |
|
|
2 |
14.85 |
98.98 |
0.11 |
|
|
|
3 |
14.93 |
99.52 |
0.07 |
|
Table 2. Acetaminophen analysis demonstrated excellent accuracy and intra-batch and inter-batch precision.
The accuracy for both concentrations and all batches was well within a very low error range, below ±2%. The intra-batch and inter-batch relative standard deviation (RSD) values were all below 0.7%, indicating significant precision.
Precise quantitation of marketed drug tablets
Paracetamol tablets of two different brands, both labeled as 500 mg/tablet, were dissolved, filtered through an Agilent Captiva Premium Syringe Filter, and analyzed. We chose regenerated cellulose as the filtration membrane because it showed excellent sample recovery for acetaminophen in a previous study [2].
As demonstrated in Table 3, sample recoveries for the two brands were 97.8% and 100.7%, both of which were well within the USP-specified range of 90 to 110%. These results capture the excellent filtration recovery of the syringe filter and the reliability of the analysis method. RSDs between three different sample batches for each brand were 0.31% and 0.17%, indicating precise quantitation.
Paracetamol |
Labeled amount per tablet (mg) |
Calculated amount (mg) |
Accuracy (%) |
RSD (%) |
|---|---|---|---|---|
|
Brand A |
500 |
488.8 |
97.8 |
0.31 |
|
Brand B |
500 |
503.3 |
100.7 |
0.17 |
Table 3. Hybrid system provided reliable accuracy and precision for paracetamol drug tablet assays.
Agilent supplies performed as well as those from original equipment manufacturer
In summary, we analyzed acetaminophen according to the USP method, and performed specificity, linear range, accuracy, precision, and recovery studies to validate the method. A Waters Alliance system was used for the HPLC analysis, and equipped with comprehensive pump and autosampler parts contained in an Agilent CrossLab performance maintenance kit. We also used an Agilent CrossLab deuterium lamp for Alliance systems and achieved excellent results, indicating that the Agilent CrossLab supplies functioned as well as the original parts.
For more details, explore Agilent Application Note 5991-6017EN. Then next time you need supplies for a Waters Alliance system, remember that Agilent CrossLab supplies provide reliable results.
References
- U.S. Pharmacopeia for Acetaminophen Tablets. United States Pharmacopoeial Convention, Rockfield, MD, USA.
- L. Zhao; W. Long. Syringe Filter Suitability for Sample Preparation in Drug Assays; Agilent Application Note 5991-2409EN, 2013.
>> Update My Profile | Subscribe to Access Agilent | Article Directory