Access Agilent eNewsletter May 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Characterize crystalline polymers with Agilent high-temperature GPC
By Adrian Boborodea
Certech ASBL, Belgium
and Alan Brookes
Agilent GPC Instrument Sales
Many polymers used in engineering applications have only limited solubility in a few solvents. This is because their high strength and toughness are usually a result of high molecular weight, high crystallinity, or both. Increasing molecular weight requires untangling the molecular chains to dissolve the material, whereas increased crystallinity requires break-up of any interchain bonds that may be present.
Polyphenylene sulfide (PPS) is an engineering polymer with a rigid backbone of alternating aromatic rings linked by sulfur atoms. It is useful as a structural material due to its high resistance to both chemical and thermal attack, and its ability to remain stiff, even at high temperatures. PPS is useful for several applications, including filter fabrics for coal boilers, felts for making paper, electrical insulation, and for manufacturing specialty membranes. PPS is naturally insulating, although the addition of a dopant makes the material semiconducting.
PPS is particularly difficult to analyze by gel permeation chromatography (GPC). The high chemical and thermal resistance of the material means that it is only soluble in specialty solvents such as 1-chloronaphthalene, at elevated temperatures above 200 °C. The combination of high temperature and the low refractive index signal (dn/dc) of polymers in 1-chloronaphthalene directed the choice of an Agilent PL-GPC 220 High Temperature GPC/SEC equipped with differential refractive index, viscometry, and light scattering detectors. This combination reliably measures true molecular weight distributions and averages. Three Agilent PLgel MIXED-B columns connected in series were used because these rugged columns exhibit excellent stability at temperatures up to 220 °C and provide resolution over the required molecular weight range. Agilent EasiVial PS-L standards were used to measure the interdetector time delay, a crucial parameter for accurate evaluation of true molecular weights using a multidetector system.
 Enlarge
Enlarge
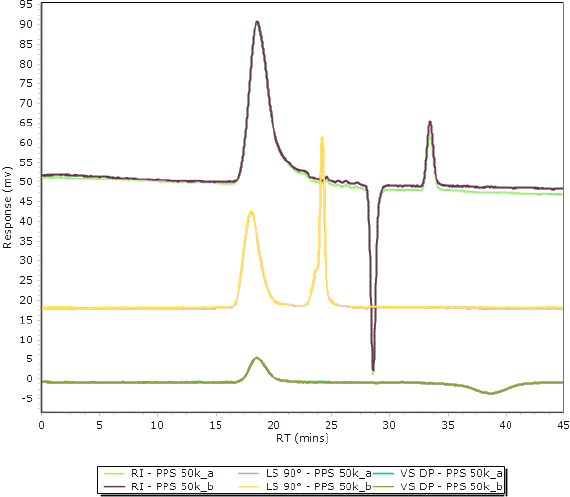
Figure 1. Overlay of two injections of polyphenylene sulfide 50k in 1-chloronaphthalene, showing excellent reproducibility for all detectors.
 Enlarge
Enlarge
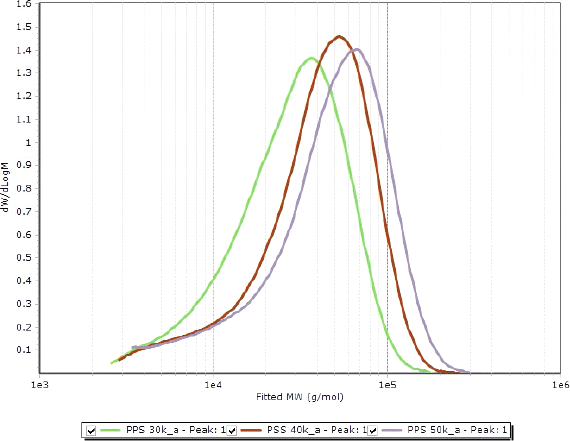
Figure 2. Overlay of molecular weight distributions of the polyphenylene sulfides, showing clear differences between the samples.
 Enlarge
Enlarge
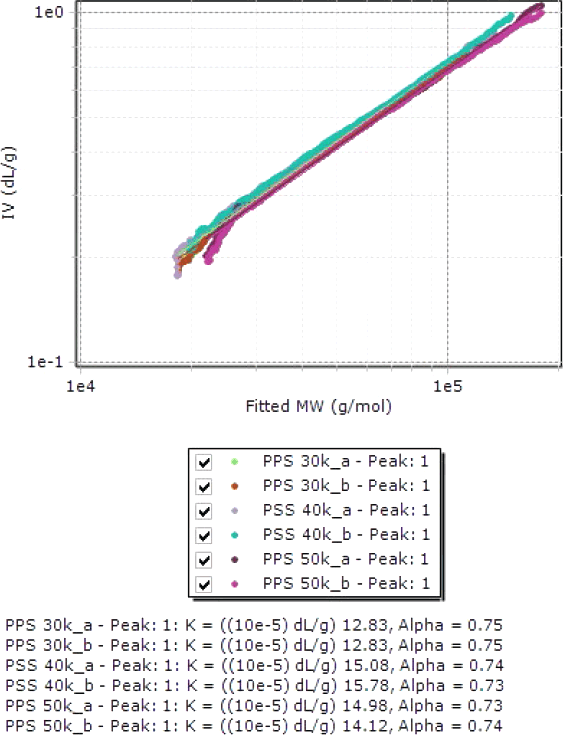
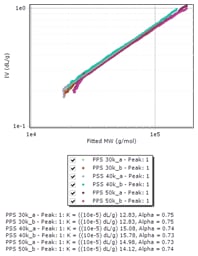
Figure 3. Overlay of Mark-Houwink plots of polyphenylene sulfides.
No degradation and excellent reproducibility
Each sample was injected twice and typical chromatograms are shown in Figure 1. Reproducibility was excellent for all detectors, which also revealed that no degradation was introduced while the samples were held in the autosampler before injection. This is because the oven of the Agilent autosampler has two temperature zones. The samples were held at 150 °C initially, and then resolubilized only 1 hour before analysis at 210 °C. This way, thermal degradation of the samples was avoided and reproducibility of results was excellent.
Clear differences in molecular weight distributions
After instrument calibration, the chromatograms obtained by triple detection were sufficient to evaluate the molecular weight distributions. A clear difference was evident between commercial PPS samples, as presented in Figure 2.
The molecular weight distributions allow calculation of average molecular weights (Mn, Mw). Table 1 shows comparisons of calculated average molecular weights from conventional calibration and triple detection GPC.
Conventional Calibration |
Triple Detection |
|||
|---|---|---|---|---|
Sample |
Mn |
Mw |
Mn |
Mw |
PPS 30k (first) |
13,300 |
30,600 |
19,600 |
34,200 |
PPS 30k (second) |
13,800 |
30,600 |
21,000 |
35,800 |
PPS 40k (first) |
16,000 |
43,500 |
25,600 |
48,100 |
PPS 40k (second) |
16,000 |
43,300 |
27,100 |
49,200 |
PPS 50k (first) |
14,500 |
49,500 |
29,400 |
58,500 |
PPS 50k (second) |
14,800 |
49,500 |
26,700 |
57,900 |
Table 1. Average molecular weights of polyphenylene sulfide, comparing GPC by conventional calibration and triple detection.
Mark-Houwink parameters reveal structure
The major advantage of triple detection is the possibility to evaluate not only the molecular weight but also the structure of the PPS samples. The Mark-Houwink plots in Figure 3 show good reproducibility and the obtained parameters are similar to those provided by the literature for linear PPS.
Accurate measurements of true molecular weights
The Agilent PL-GPC 220 High Temperature GPC system, with triple detection – combined with Agilent PLgel MIXED-B columns, is a reliable system for accurate measurements of the true molecular weights of commercial polyphenylene sulfides. Moreover, it allows evaluation of the Mark-Houwink parameters of PPS samples, providing critical information on sample structure. It also shows excellent correlation with the literature reference values for linear PPS. The molecular weight distributions allow calculation of all the average molecular weights.
Full details of this analysis are freely available in Agilent publication 5991-5570EN [1].
Agilent offers a wide array of solutions for engineering polymers
Reliable analyses of polymer manufacturing materials are vital to ensure product performance. Due to the importance of efficiency and cost-effectiveness, it is essential to run accurate analytical processes that can quickly ascertain quality. The Agilent GPC/SEC portfolio offers market-leading instrumentation, columns, standards, and data analysis software for all types of polymer analysis.
What’s more, you can easily find the best Agilent column and sample prep products for any application with Agilent’s handy online selection tool, the LC Navigator. You can also receive a free copy of Agilent’s easy-to-understand GPC/SEC Wall Chart. When it comes to polymer analysis, Agilent is your go-to source.
Reference
- Boborodea, A; Brookes, A. “Characterization of Polyphenylene Sulfide Using Gel Permeation Chromatography with Triple Detection” Int. J. Poly. Anal. Char. 2014, 20, 172-179.
>> Update My Profile | Subscribe to Access Agilent | Article Directory