Access Agilent eNewsletter May 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Ask the Expert: What are the first steps in biosimilar characterization?
By Koen Sandra
Research Institute of Chromatography, Belgium
and Maureen Joseph
Agilent Bipharma Applications
Therapies derived from biopharmaceuticals such as monoclonal antibodies (mAbs) have dramatically changed many peoples’ lives, and the potential for new treatments – even cures for some of our most intractable diseases – is boundless. In a new series of articles, our experts explore the steps needed to develop an effective biosimilar, looking at protocols for Herceptin. The techniques discussed are not very different from those used to develop and evaluate innovator drugs.
This introductory article looks at the first steps in mAb biosimilar characterization using affinity chromatography, to be followed by articles on reversed-phase liquid chromatography of intact and fragmented mAbs and glycan, charge variant, and aggregate analysis. A final article will introduce some of the powerful software tools that guide your journey to successful biosimilar development. In addition, you can explore our complimentary E-Book Prepping Biosmilars, which contains all of the articles in greater detail.
Q. What are mAbs?
Answer: mAbs are glycosylated macromolecules binding a specific target (antigen), blocking its function and inducing an immune response against the target cell. mAbs are a rapidly growing class of therapeutics with increasing numbers of these drugs being approved. Top-selling mAbs are or will soon be available to the market as “generics” – driving an explosion of interest in biosimilars. The complexity of these molecules and their sensitivity to changes in the manufacturing process creates an increased need for advanced analytical techniques to thoroughly characterize and compare biosimilar versions to originator drugs.
Q. What techniques are best for analyzing complex mAbs?
Answer: Chromatography and mass spectrometry are powerful analytical tools for examining mAbs. Reversed-phase liquid chromatography (RPLC) and liquid chromatography mass spectrometry (LC/MS) are used for studying primary structures and post-translational modifications. Size exclusion chromatography (SEC) is applied to assess aggregation, and ion-exchange chromatography (IEX) to examine charge variants. For glycan analysis, hydrophilic interaction chromatography (HILIC) is becoming the technique of choice.
Before these additional chromatographic techniques can be used, one first needs to isolate these recombinantly produced mAbs from cell-culture supernatants. This is typically done with affinity chromatography, for example using an Agilent Bio-Monolith Protein A column.
Q. Why is affinity chromatography important?
Answer: At the analytical scale, affinity chromatography is used early in the development of monoclonal antibodies for the high-throughput determination of mAb titer and yield directly from cell-culture supernatants. Affinity chromatography also enables early assessment of biocomparability, and purifies µg amounts of material for further measurements, for example by mass spectrometry (MS) or by other chromatographic techniques such as RPLC, SEC, and IEX.
Q. How do I determine mAb titer of different clones?
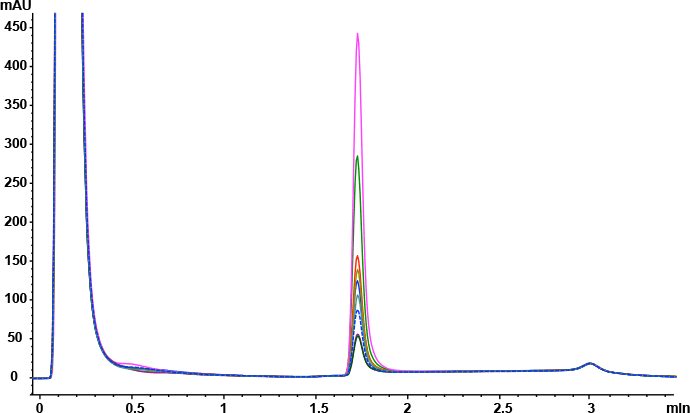
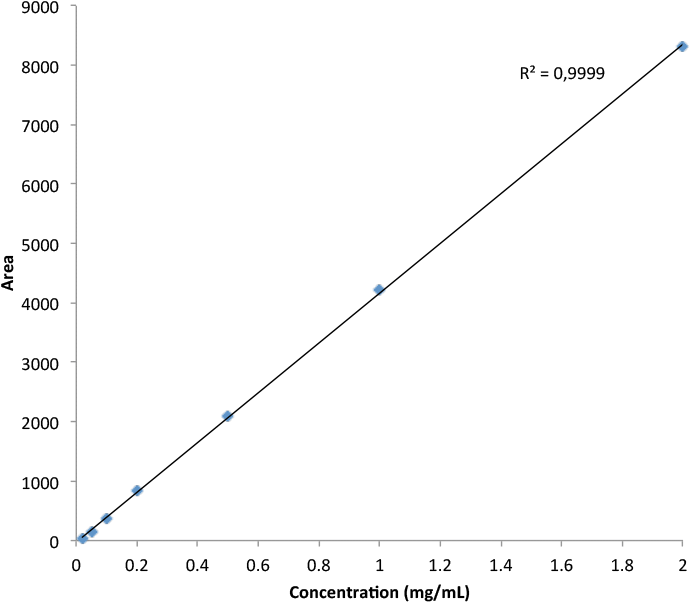
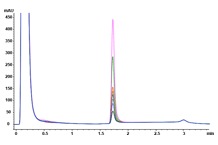
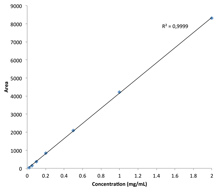
Answer: The Agilent Bio-Monolith Protein A column possesses all the characteristics one needs to determine mAb titer in cell culture supernatants. It is fast, precise, and linear in the expected mAb concentration range. Figure 1 shows the analysis of nine trastuzumab-producing CHO clones using the Agilent Bio-Monolith Protein A column. mAb titer can be measured against a calibration curve established using a dilution series of Herceptin originator (Figure 2). Absolute mAb concentrations are shown in Table 1.
CHO clone |
Concentration (mg/mL) |
|---|---|
3 |
0.210 |
8 |
0.256 |
9 |
0.494 |
10 |
0.757 |
24 |
0.262 |
25 |
0.098 |
26 |
0.090 |
28 |
0.173 |
32 |
0.144 |
Table 1. Absolute mAb concentrations determined in the different trastuzumab-producing CHO clones.
 Enlarge
Enlarge
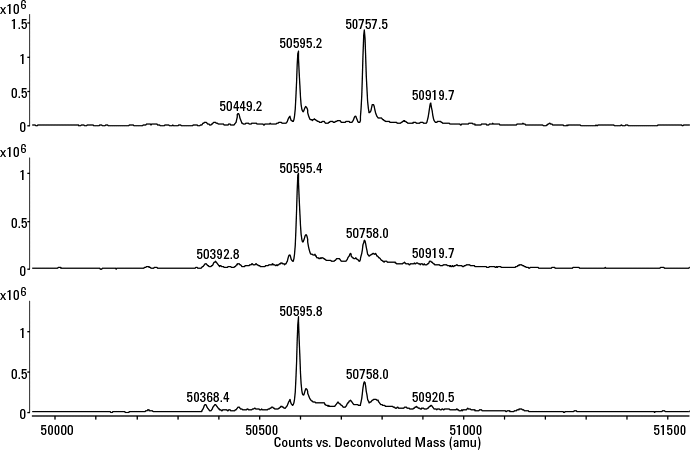
Figure 3. Deconvoluted heavy chain spectra of a Herceptin originator and two trastuzumab-producing clones. G0, G0F, G1F, and G2F refer to the N-glycans attached to the mAb backbone.
 Enlarge
Enlarge
Figure 1. Overlaid Agilent Bio-Monolith Protein A chromatograms of nine trastuzumab-producing CHO cell clones using citric acid as the elution buffer.
 Enlarge
Enlarge
Figure 2. Herceptin Protein A calibration curve (0.02 to 2 mg/mL).
Q. How do I compare the biosimilar to the originator molecule?
Answer: Begin by assessing the structural characteristics of the preferred clone, typically the high titer clones, and compare these with the originator molecule. Protein A fractions are collected and measured by high-resolution MS, following disulfide bond reduction, giving rise to the light and heavy chains. This strategy lets you verify the amino-acid sequence and reveals the glycosylation pattern. To reduce the mAb directly in the collection vial containing acidic buffer, TCEP is typically chosen due to its reducing capacities over a broad pH range. Reduced fractions are delivered to the MS system following online desalting on an Agilent PLRP-S for Biomolecules, 1000Å, 2.1 x 12.5 mm column. By comparing the MS profiles of the heavy chain of the Herceptin originator and two high producing clones (Figure 3), it can be seen that the products are similar from a qualitative perspective, yet differ quantitatively in the glycosylation profile, i.e. the clones are undergalactosylated. The LC/MS used in this example is an Agilent 1290 Infinity Binary LC coupled to an Agilent 6545 Ultra High Definition Accurate-Mass Q-TOF.
The Agilent Bio-Monolith Protein A column can then be used to guide cell culture optimization, evaluating changes in titer with tuning of the cell culture media to bring the glycosylation profile of the biosimilar to within the specifications of the originator. Optimization can, for example, be done by feeding with uridine, galactose, and manganese chloride at different concentrations. These are the substrates and activators of the galactosyltransferase responsible for donating galactose residues to G0F and G1F acceptors.
Agilent offers a wide spectrum of biopharma solutions
At every stage in the process, from disease research to QA/QC and manufacturing, for moving therapeutics successfully to market, Agilent can help you make the right choices. And it’s not just because we build reliable instruments that provide accurate, reproducible results. We understand the biopharmaceutical workflow and provide families of products that work together seamlessly – as engines of research, discovery, and development – to move candidate biopharmaceuticals forward. Contact an Agilent Representative today to learn more.
>> Update My Profile | Subscribe to Access Agilent | Article Directory