Access Agilent eNewsletter, January 2014
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Water, water everywhere… but is it fit to drink?
By Joe Weitzel
Agilent Global Environmental Manager
Obtaining the highest level of quality and purity in drinking water is essential, whether the source is bottled water or the kitchen faucet. However, industrial contamination or improper disposal practices can lead to trace contamination that can have significant effects on the quality of our water. Accurately monitoring the levels of contaminants in treated water, wastewater, surface water, and ground water is essential for protecting both human and environmental health.
Because ensuring the quality and safety of water is so important, Agilent offers many solutions for assessing water quality. In this article we explore a couple of recent examples.
Analyte protectants can improve chromatographic results
When performing trace GC/MS analysis, ensuring the integrity of the analytes is essential. In an examination of ground and waste water from California, Eduardo Morales at Weck Laboratories investigated the matrix effects and the instability of organophosphorus pesticides in various solvents, which can lead to skewed results (see Agilent Application Note 5991-1808EN).
The quantitation of pesticides residues can be adversely affected by a phenomenon known as the matrix-induced chromatographic response enhancement effect. This effect is evident in improved chromatographic peak intensity and shape when affected compounds are injected in the presence of a complex matrix. Matrix components mask potentially active sites in the GC flow path, leading to fewer of these sites being available to interact with analytes, and thus fewer losses of analytes and better peak shapes.
One approach to compensate for the matrix effect is to use analyte protectants that bind to potential active sites in the GC flow path and inhibit their interference in the analysis. In this way, the benefit of matrix-induced chromatographic enhancement (larger and sharper peaks) can be attained without the risk of matrix interferences.
 Enlarge
Enlarge
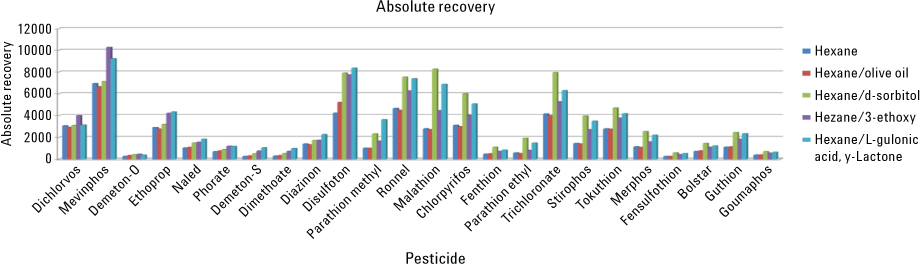
Figure 1. Comparison of recoveries using several different analyte protectants and hexane as the extracting solvent.
Active sites may occur within the GC flow path as free silanols – collecting within poorly or nondeactivated glass liners, in poorly or nonuniformly coated open-tubular columns that expose the fused silica, or even in some hardware interfaces. The use of analyte protectants that are generally rich in polar hydroxyl groups can mitigate these effects. While extracted blank matrix can also be used for this purpose, it can be time-consuming and costly. In addition, some pesticides are not stabile in some of the common extraction solvents. Certain organophosphorous pesticides stored in ethyl acetate solutions were seen to degrade over time. After the evaluation of several extraction solvents, hexane provided the best analyte stability for a wide range of organophosphorous pesticides during GC/MS analysis. When using hexane for extracting the analytes from water, of the protectants tested, d-sorbitol offered the most benefits to trace pesticide recovery (Figure 1). An Agilent J&W HP-5ms Ultra Inert GC column fitted to an Agilent 7890/7000 Triple Quadrupole GC/MS System was used for this testing.
Greater speed, accuracy, and flexibility for EPA Method 8270
Dale Walker, an applications chemist at Agilent, wanted to demonstrate the use of the Agilent 5977A Series GC/MSD coupled with the Agilent 7890B Gas Chromatograph to meet the performance challenges of the USEPA method 8270. (Refer to Agilent Application Note 5991-2153EN). Many environmental laboratories strictly follow this method to identify and quantitate a large number of trace semi-volatile compounds in environmental matrices. The compounds include polynuclear aromatic hydrocarbons, chlorinated hydrocarbons, pesticides, aldehydes, nitrosamines, phthalate esters, and phenols. Typically laboratories analyze more than 70 compounds in a single analytical run, at a working concentration range between 20 to 160 ppm. In addition, a growing number of laboratories are pushing for even lower detection limits and a wider dynamic range. As a result, instrumentation, technologies and techniques must continue to evolve to meet these growing analytical demands.
 Enlarge
Enlarge
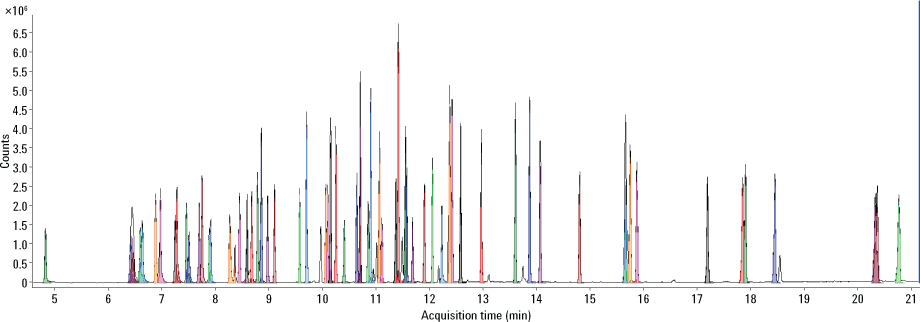
Figure 2. Total compound chromatogram of 20 ppm standard mix on an Agilent J&W DB-UI 8270D GC column.
In this example, an Agilent J&W DB-UI 8270D column was chosen for the analytical separation. Results showed good performance for all compounds specified by Method 8270 with regard to sensitivity and linearity. The calibration range of 0.2 to 100 ppm was well below what has been historically used by US EPA laboratories, and all 86 compounds, internal standards and surrogates were off the column in less than 21 minutes (Figure 2). This fast result is important for environmental contract labs trying to increase sample throughput. What’s more, the Agilent MassHunter data system brought added value and flexibility to routine analysis. MassHunter also provided visual indicators to supplement the numerical values typically used for the pass/fail criteria defined by Method 8270. Use of the new extractor lens and Etune tuning procedure offered superior performance and reliability.
More environmental solutions from Agilent
These are just two examples derived from Agilent’s 40 years of environmental analysis and regulatory expertise. If you are involved in the measurement of organic and inorganic chemicals in water, soil biota, or air, Agilent has the right solution for you. Our instruments, accessories, consumables, and services are designed specifically to meet your needs. Take a moment to learn more about how Agilent can help you achieve outstanding results.
>> Update My Profile | Subscribe to Access Agilent | Article Directory