Access Agilent eNewsletter, October 2013
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Understanding GC columns – the heart of gas chromatography – Part 2
By Allen K. Vickers, Daron Decker, Ronald E. Majors
Agilent GC Applications
Last month in Part 1 of this overview we focused on the aspects of GC column development, manufacture, and testing. In Part 2, we’ll talk about stationary phase coating and quality control.
The importance of stationary phase coating
The stationary phase determines chromatographic resolution and reproducibility, making the coating step important to the overall effectiveness of the column and to its particular use. The phase should be uniform in thickness, homogeneity, and adherence to the inner wall. This ensures there is no disruption during programmed temperature cycles or injection of solvent and sample. Modern phases are usually immobilized by cross-linking, which gives them added stability over non-cross linked phases, and allows solvent washing of contaminated columns after they are put into use.
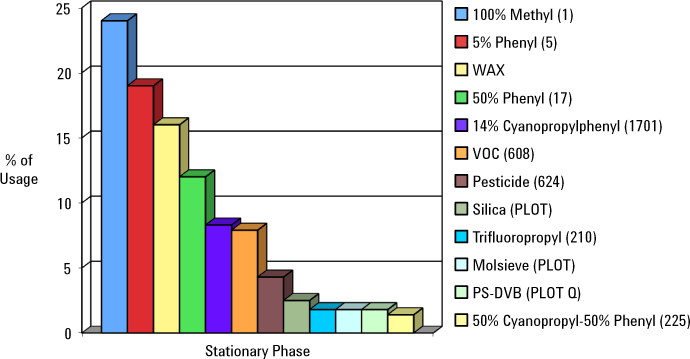
The application dictates the type of stationary phase. Popular stationary phases are shown in Figure 1.
Most stationary phases are polymeric. To ensure that the polymer used for GC column coating today matches the polymer used months and years from now, many manufacturers will synthesize, purify, and pretest it before using it for column coating. For copolymers, it is important to control the substitution percentage to ensure reproducible retention. It is especially critical to remove lower molecular weight fractions since these can cause column bleed, particularly at high temperature. Once the polymer is deemed acceptable, it should be mixed in a suitable solvent at the correct concentration because this composition determines the film thickness of the stationary phase.
Various methods and steps go into successful coating
The methods for coating GC capillary columns are dynamic or static. Table 1 shows how these two processes work to deposit the coating, what influences the film’s thickness in each method, and the uses and advantages of each process.
Type of coating |
Method |
Film deposition |
Film thickness |
Advantage |
Used for |
|---|---|---|---|---|---|
Dynamic |
Plug of solvent containing stationary phase introduced at the beginning of the column and is forced through under pressure at constant speed. |
Coating is left behind as plug moves through the column. |
Influenced by solution composition, physical properties of surface, stationary phase and solvent, among other factors. |
Speed of manufacture. |
Mainly PLOT columns, rarely for WCOT capillaries. |
Static |
Column filled with stationary phase dissolved in volatile solvent at a concentration appropriate for desired film thickness. One end is then sealed and the tube evacuated. |
Coating is deposited from the solvent front as it retreats backwards down the column. |
Proportional to concentration of stationary phase in solvent, and tubing diameter. |
Column reproducibility. |
WCOT capillaries. |
Table 1. Stationary phase coating methods
The stationary phase can be immobilized or cross-linked in situ during coating using either the dynamic or static coating method. This process must be performed in the absence of oxygen to ensure the development of a stable, inert phase. In another approach, polymeric stationary phases can be formed on the wall surface by first depositing the monomers and then starting polymerization by heat or catalysis. This process locks the stationary phase on the inner wall and immobilizes it. It also works with either the dynamic or static method of application.
Whatever process is used for coating, it is important to control the phase ratio (β) regardless of the column’s inner diameter (id). If there are id differences between different columns, then the phase ratio is adjusted by modifying the film thickness to maintain constant β. Typical tolerance of capillary tubing is +/- 6 µm, a 1.1% variation for a 0.53 mm column but a 2.4% variation for a 0.25 mm column.
Once the stationary phase is bonded or immobilized, any unreacted polymer must be removed by rinsing and followed by removing the rinsed solvent. The solvent is removed by slowly heating the column while an inert gas flows through it. As a final step, the column is heated to its upper limit while oxygen-free gas is flowing through it.
Controlling quality and assurance for proven column-to-column performance
Rigorous quality testing ensures column-to-column performance, so it is important to use meaningful test probes to assess bleed, theoretical plate number, and retention indexes. Table 2 provides a list of typical test probes. Of course, it is important to remember that no single set of probes is sufficient to test all columns for all applications. For very demanding applications, a “premium” column, such as a mass spectrometry (MS) phase, will generally have more test probes and perhaps stronger, active probes in the test mix compared to a “standard” column of similar design. Premium and “specialty” application columns such as semivolatile or PONA columns may also be put through a second quality control test with even tighter specifications for target analytes.
Compound class |
Representative test probe |
Purpose |
|---|---|---|
Hydrocarbons |
Undecane, tridecane, tetradecane, pentadecane |
Efficiency and retention |
Alcohols |
1-Dodecanol |
Activity, retention index, peak height ratio |
FAMEs, PAHs |
Methyl caprate, acenaphthylene |
Retention indexes |
Acids |
4-Chlorophenol |
Acidic character, peak height ratio |
Bases |
1-Decylamine |
Basic character, peak height ratio |
Table 2. Test probes for column characterization
In all cases, columns should be tested using isothermal conditions. This is a more rigorous test than temperature programming, which tends to sharpen peaks and hide problems.
Column efficiency is measured in plates per meter for a well-retained compound such as pentadecane with a k value greater than 5. Choosing a low k value enhances the plate count and is not a true test of column efficiency.
Selectivity measurements are provided using different probes with different functional groups. Inertness is measured with probes that have non-sterically hindered functional groups. This allows maximum interaction between stationary phase and capillary walls. The best measure of inertness is the peak height ratio between a hydrocarbon and a compound with a polar functional group. Hydrocarbon peaks are not affected by active surfaces whereas polar compounds can be. Peak height ratios for 1-dodecanol/tetradecane, 1-decylamine/tridecane, and 4-chlorophenol/tridecane are good measures of column inertness.
To obtain a bleed profile, a temperature program is run from 50 °C to the column’s maximum operating temperature. For a typical GC/MS, 5% diphenyl/95% dimethylpolysiloxane column, bleed specification is below 4 pA using a flame ionization detector over this temperature range.
Specialty columns demand application-specific testing
Application-specific columns – such as pesticide columns or semivolatile columns used for environmental methods – are usually tested with target compounds and a detector typical of the application. With chlorinated pesticides, for example, an electron-capture detector would be used with a sample containing CLP test pesticides from the Environmental Protection Agency Method 608 or 8081A list. For primary and confirmation dual columns, such as those used in blood alcohol analysis, each column is tested individually to ensure that it meets demanding separation challenges such as the ethanol/acetone pair.
Find the right column for your application
WCOT capillary GC columns provide trouble-free operation and reproducible, reliable separations with rigidly controlled manufacturing and quality control. Agilent columns are tested thoroughly with demanding test probes and a data sheet is shipped with every column to provide assurance that the column will perform as expected.
Whatever your GC requirements, Agilent manufactures a full range of Ultra Inert, capillary, packed, low-bleed GC/MS, polysiloxane, PEG, specialty, and PLOT GC columns. Explore the whole Agilent GC column family here.
This article is adapted from Vickers et al. “The Art and Science of GC Capillary Column Production”. LC.GC. July 1, 2007.
References
>> Update My Profile | Subscribe to Access Agilent | Article Directory