PD-L1 IHC 22C3 pharmDx testing for Cervical Cancer

PD-L1 is a proven biomarker for patient response to KEYTRUDA in cervical cancer
- Cervical cancer is the third most common gynecologic cancer in the United States
- Patients with metastatic cervical cancer have poor prognosis—the median overall survival is only one year, with short-lived responses to treatment and rapid deterioration of quality of life
- Anti-PD-1 therapies are a promising new treatment class for many cancer types—the KEYNOTE-158 clinical trial studied the efficacy of KEYTRUDA, an anti-PD-1 monotherapy, in patients with recurrent or metastatic cervical cancer
- IHC testing for PD-L1 expression levels enables identification of patients most likely to benefit from anti-PD-1 monotherapy
Proven in KEYNOTE-158 for PD-L1 results in cervical cancer that you can rely on
PD-L1 IHC 22C3 pharmDx was the only IHC assay used to assess patients for PD-L1 expression in the KEYNOTE-158 trial
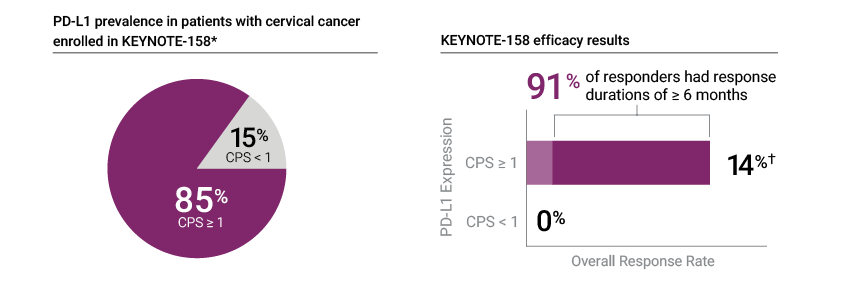
* Prevalence calculation based on patients with known PD-L1 expression (Cohort E, n=97)—1 patient (1% of the cohort) had unknown PD-L1 expression status; † Efficacy results based on patients in KEYNOTE-158 Cohort E with tumors that expressed PD-L1 with a CPS ≥ 1 and who received at least one line of chemotherapy in the metastatic setting (n=77)

Because durations of response are short at first line and there is no standard of care at second line, it is critical to assess PD-L1 expression status upon diagnosis
- Platinum-based chemotherapy regimens are the most common first-line treatment for metastatic cervical cancer
- However, treatment efficacy is variable, with patients experiencing progression-free survival (PFS) of less than one year
- Additionally, following first-line treatment, there is no universally accepted standard of care, and PFS is even shorter, typically less than six months


KEYTRUDA® is a registered trademark of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA
References: 1. PD-L1 IHC 22C3 pharmDx [package insert]. Carpinteria, CA: Dako, Agilent Pathology Solutions; 2018. 2. Keytruda [package insert]. Kenilworth, NJ: Merck & Co., Inc.; 2018. 3. Reddy OL, Shintaku PI, Moatamed NA. Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagn Pathol. 2017;12(1):45. 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. 5. Borcoman E, Tourneau CL. Pembrolizumab in cervical cancer: latest evidence and clinical usefulness. Ther Adv Med Oncol. 2017;9(6):431-439. 6. Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, for ESMO Guidelines Committee. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv72-iv83. 7. Boussios S, Seraj E, Zarkavelis G, et al. Management of patients with recurrent/advanced cervical cancer beyond first line platinum regimens: Where do we stand? A literature review. Crit Rev Oncol Hematol. 2016;108:164-174.
For countries outside of the United States, see the local KEYTRUDA product label for approved indications and expression cutoff values to guide therapy.
D49166/06.1