Access Agilent eNewsletter May 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Harness the power of GC/MSD to characterize functional foods
By John Lee
Agilent Food Market Manager
Many botanical products are available as teas, capsules, or tablets (i.e. dietary supplements). These products are perceived to be health promoting and their effects are often associated with defined compounds or classes of compound. Botanicals can, however, also contain other compounds that are not so desirable, and that may have unintended and detrimental health effects. It is important to characterize botanicals to ensure that they are free from these types of compound and so protect the health of consumers.
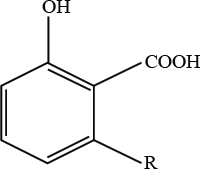
R = C13H27 (C13:0)
R = C15H31 (C15:0)
R = C15H29 (C15:1)
R = C17H33 (C17:1)
R = C17H31 (C17:2)
Figure 1. Structure of ginkgolic acid.
 Enlarge
Enlarge
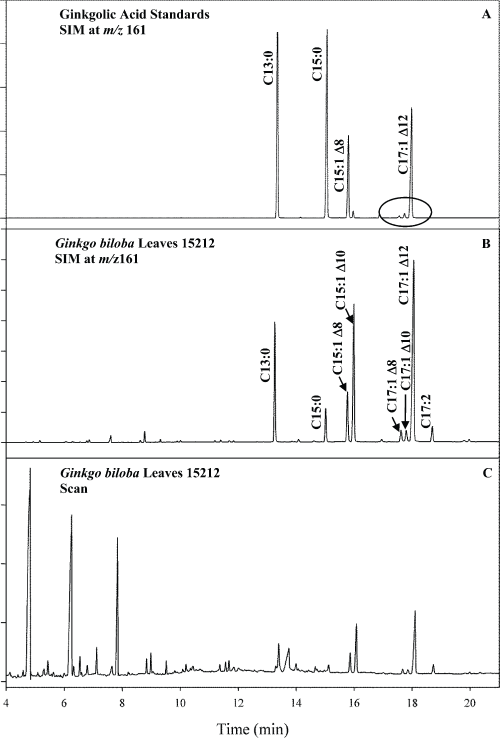
Figure 2. SIM and scan GC/MS chromatograms of ginkgolic acid standards and sample 15212 of Ginkgo biloba leaves.
 Enlarge
Enlarge
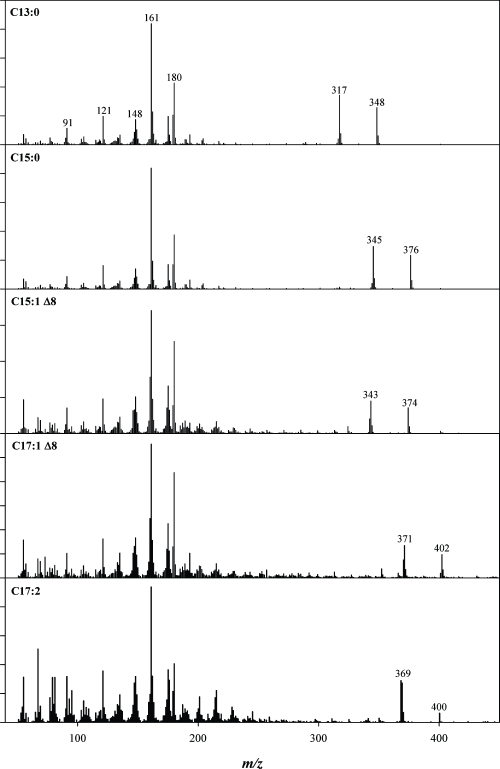
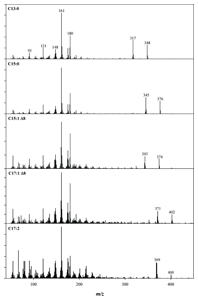
Figure 3. EI-MS spectra of ginkgolic acids.
In this example, Wang, M. et al. [1] characterized and quantitatively analyzed ginkgolic acids (GAs) in Ginkgo biloba samples (Figure 1). Ginkgo biloba is used worldwide as a tea and dietary supplement, but has been thought to cause adverse effects, such as contact allergic dermatitis and possible mutagenesis. Gas chromatography, with single quadrupole mass spectrometry (GC/MSD), was used for the analysis of GAs because it offers a number of benefits over other techniques for this application.
Benefits of EI and SIM
An electron ionization (EI) source provides reproducible compound fragment ions during the ionization process. When exploring a particular class of compound, EI can be helpful because many diverse structures can be fragmented down to a core structure. Hence, a very sensitive and specific probe ion can be chosen for selected ion monitoring (SIM) during chromatography. A probe ion can then pick up many analogues of a compound class, even ones that were unexpected (note the example shown in section B of Figure 2).
Benefits of high-resolution gas chromatography
Finding the different analogues of a class then requires good separation of all potential analogues and the interferences from background matrix. In botanicals, this can be challenging. HPLC is a common technique used to assess GAs. However, GC/MSD is becoming increasingly popular because of the very high resolution and selectivity of gas chromatography. Indeed, the performance is sufficient to distinguish even between positional isomers of these compounds and to differentiate the carbon number of side chains. This capability is unique and ensures accurate quantitation of target compounds and reliable discovery of new compounds (see Figure 2B).
Benefits of SIM/SCAN collection
It is desirable to have a way of verifying the identity of a chromatographic peak. For calibrated compounds, a very strict matching of retention time should be expected against a standard. However, there is often a need for further verification of both calibrated and uncalibrated compounds. For these reasons, many analysts run the GC/MSD in what is called SIM/SCAN mode. This mode of data collection provides the sensitivity of SIM mode for the probe ion and simultaneously provides a scan to allow identification of a chromatographic peak through its full EI spectrum. Such identification is possible by matching the spectrum to those of a library of previously isolated compounds. The Agilent GC/MSD software lets you access a wealth of libraries that contain such information, and also create your own library entries for new compounds that you discover. The beauty of EI is that often it produces rich and diagnostic fragmentation that is different for each molecule. For GAs, this is illustrated in Figure 3.
GC/MSD techniques and tools for food safety assessment
Figures 2 and 3 come from the work of the National Center for Natural Products Research, at the University of Mississippi. Gas chromatography was performed on an Agilent 7890B GC system fitted with an Agilent J&W HP-88 GC column, linked to a 5975C GC/MSD for mass spectrometry. Derivitization was required with TMSO (trimethylsulfonium hydroxide) but this was in no way a hardship since this reagent is simply added (50 µL) to the extract before injection. For routine analysis, this step can be automated using the 7890 autosampler. Derivatization not only helps the GC separation, but also aids in the structurally significant fragmentation of the molecule in the mass spec, which is diagnostic for identity. This system is similar in cost to high-end UHPLC systems.
Recapping, Figure 2 shows selected ion chromatograms at m/z 161 for a mixture of four ginkgolic acid standards (A) and the plant sample 15212 (ginkgo leaves) (B), and the total ion chromatogram for this sample (C). The component pairs – C13:0/C15:1 and C15:0/C17:1 – that were difficult to resolve by HPLC were clearly resolved with this technique.
Figure 3 shows the electron ionization spectra of five analyzed ginkgolic acids. For more information on this technique read Agilent Application Note 5991-5634EN.
Agilent offers a broad array of solutions for food safety
There are many other organic compounds of concern in botanical and dietary supplements, such as pesticide residues. GC/MS and LC/MS also offer ways to determine pesticides. LC/MS is also used for looking at pyrrolizidine alkaloids and N-oxides in botanicals.
These techniques are also important to characterize efficacious components in botanicals and to authenticate the botanicals themselves. Future articles will illustrate the use of LC/MS and GC/MS for these applications, in addition to various atomic techniques such as ICP-MS, ICP-OES, and MP-AES.
In all cases, food products are some of the most challenging matrices to analyze. Agilent’s range of industry-leading food safety solutions covers all areas of supplement and compound analysis. Contact your Agilent Representative to learn more today.
Reference
>> Update My Profile | Subscribe to Access Agilent | Article Directory