Access Agilent eNewsletter May 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Agilent microRNA analysis detects blood doping in athletics
By Francesco Donati
Laboratorio Antidoping FMSI, Rome
and Bernhard Wuest
Global Marketing Manager Sports-Doping / Sports-Medicine
Some athletes use unethical means to gain an advantage and improve their performance by using blood transfusions, which increase the rate of oxygen transport in the body. The World Anti-Doping Agency has banned such blood doping.
There are two types of blood transfusion. Homologous transfusion uses the blood of the same individual, whereas autologous transfusion uses blood from a donor. There is a method for detecting homologous blood transfusion used by antidoping laboratories, but there is no internationally recognized method for the direct detection of autologous blood transfusions. At present, autologous transfusion can only be detected indirectly by targeting longitudinal profiling of key hematological parameters. In this article, we examine methodologies to address this challenge.
MicroRNA as biomarkers for blood transfusion
We looked at the role and variability of microRNAs (miRNAs) as biomarkers to provide evidence of autologous blood transfusion. miRNAs are noncoding RNAs, 18 to 24 nucleotides long, that act as posttranscriptional modulators of mammalian gene expression. They are produced in the nucleus by a coding gene as pri-miRNA, exported to the cytoplasm as pre-miRNA, cleaved by a dicer enzyme, and then processed by a RISC (RNA-induced silencing complex) to the mature functional miRNA. Mature miRNAs are involved in the regulation of many physiological processes (such as erythropoiesis), since they can affect the cleavage of a target messenger RNA (mRNA), function as transcriptional repressers, or are involved in mRNA deadenylation.
Six whole blood samples were extracted from healthy athletes at three different times (T=0 within 24 h of sample collection, T=1 after 15 days, T=2 after 30 days) and quantified (mi923, mi150, mi144, mi96, mi196a, mi30b, mi197, mi451). Another blood sample was withdrawn fresh from a donor, stored as concentrated erythrocytes and, after 30 days, used to provide an ex-vivo autologous blood transfusion with new fresh blood from the same donor. All miRNAs were extracted with a specific kit and quantified with an Agilent 2100 Bioanalyzer chip electrophoresis system. The miRNA samples (5 ng) were then retrotranscribed to cDNA, which were used as templates for quantitative PCR (qPCR). Expression of miRNAs was calculated as relative quantities (RQ) estimated as 2-ΔΔCt [the ΔΔCt value was obtained as normalization of ΔCt sample to the ΔCt (count) of a sample used as calibrator].
 Enlarge
Enlarge
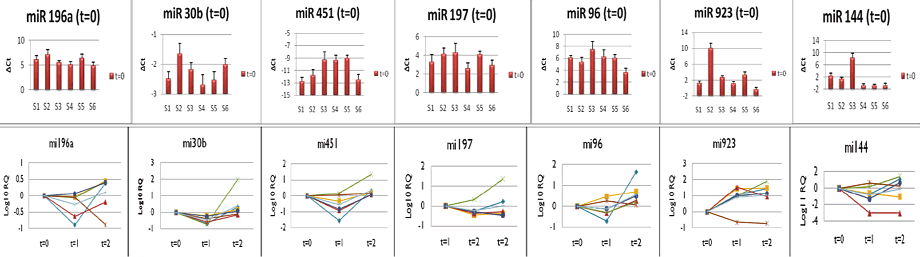
Figure 1. Variability of miRNA expression in blood samples (above). Longitudinal variability of miRNAs over 30 days of storage (below).
 Enlarge
Enlarge
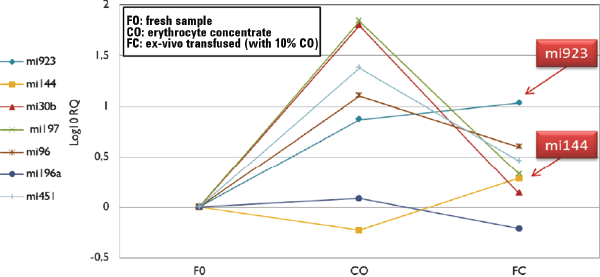
Figure 2. Results of variation in the expression of miRNA after an ex-vivo autologous transfusion.
Detecting changes to miRNA over time
The results of our forensics testing can be seen in Figure 1. If you look at the upper row of the graphic, you will see the variability of the miRNAs. At T=0, mi144, mi923, and mi451 showed the most consistent variability in the RQ, while mi30b, mi196a, mi197, and mi196 were the least variable. The lower section of the graphic shows the longitudinal variability of miRNA over 30 days of storage.
There was a gradual tendency of the miRNAs to increase their expression in samples at T=2 compared to T=0. The variability of mi197, mi96, mi196a, mi30b, and mi451 was limited to a 2-4 order of magnitude. Differences in expression of mi144 and mi923 were most consistent from T=0 to T=2, though with great variability between samples.
Providing evidence of autologous blood transfusion
We observed a marked difference in the erythrocyte-concentrate sample (T=30), where expression levels of mi923, mi30b, mi197, mi96, and mi451 was higher compared to the fresh samples at T=0. Interestingly, the expression of some miRNAs was very high and were also detectable in the ex-vivo transfused sample with higher levels than the fresh nontransfused sample. Moreover, mi144 and mi923 show the most significant increase in the transfused sample compared to the fresh one. It therefore seems possible to use miRNA expression both as biomarkers of storage and biomarkers as evidence of blood doping detection (Figure 2).
Using expression of miRNAs seems to be very promising. The technique has the sensitivity and reproducibility required for this application. However, one of the key objectives of future work is to examine intra-individual variability in the expression of the diagnostic miRNAs. The next steps for our research group include broadening the panel of miRNAs assayed and carrying out a solid population study, with a statistical and forensic analysis of variability. Read the full story of this work in Agilent publication 5991-5717EN.
Agilent solutions for a level playing field
Ensuring that participation in sport remains fair is important to fans, sponsors, and most competitors. However, athletes in all areas of sport continue to use a wide and growing array of illegal and performance-enhancing drugs and blood products. Agilent is proud to help combat these threats to fair competition by working with regulatory agencies and event organizers of competitions at many levels, including the Olympics and the Pan-American Games. Agilent has a wide variety of sample prep, GC and LC columns, and instruments for Gas Chromatography, Liquid Chromatography, and Mass Spectrometry that can aid in blood testing.
To discover more Agilent solutions that can assist you in your particular applications, contact an Agilent Representative today.
>> Update My Profile | Subscribe to Access Agilent | Article Directory