Access Agilent eNewsletter, March 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Quickly, easily, and accurately analyze mycotoxins in food with Agilent LC/MS solutions
By John Lee
Agilent Food Market Manager
Tandem mass spectrometry (MS/MS) is often used for complex food matrices because of its selectivity, sensitivity, and linear quantitation range. In this article we will explore the use of LC/MS/MS for the analysis of mycotoxins in foods, from quantitation of regulated compounds through to screening of many compounds, using minimal sample prep.
Mycotoxins are secondary metabolites produced by fungi such as Fusarium, Aspergillus, and Penicillium. These fungi are often present on plant products, and can proliferate if food is poorly stored. Many mycotoxins are carcinogenic, and they spoil millions of tons of cereals and other foods every year, with estimated annual losses in the US of $1 Billion. The primary mycotoxins regulated in the EU and US are aflatoxins, deoxynivalenol, fumonisins, ochratoxin A (EU), zearalenone, and patulin. Globally, there are hundreds of potentially toxic fungal strains and metabolites that also warrant surveillance.
Regulated mycotoxins
Traditional HPLC methods with MS or fluorescence detection can involve time consuming sample prep to achieve low levels of detection for regulated control. Additionally, sample prep is usually directed to a limited number of compounds and so may require more than one workflow to cover all mycotoxins relevant for a particular food type.
 Enlarge
Enlarge
Figure 1. Coelution of target mycotoxins and their internal standards – dotted lines are labeled compounds and solid lines are the native (or target) compounds.
Quantify mycotoxins in maize with minimal sample prep
In our first example, an Agilent 1290 Infinity LC system was coupled to an Agilent 6490 Triple Quadrupole LC/MS at the Center for Analytical Chemistry (Tulln, Austria). Their fast method analysis quantitated all mycotoxins regulated in cereals. The team used labeled internal standards to enable a simple solvent calibration with simple extraction, no clean-up, and hence low running costs. Figure 1 shows elution of target mycotoxins and their internal standards, with a fast run time of under 10 minutes. Three compounds coeluted at the end of the run. Fast polarity switching allowed the selection of the optimum ion signal for all compounds (zearalenone responds best as a negative ion whereas the other two aren whereas the other two areions).
Although the aflatoxins had different masses, complete separation was required because some masses were the same as other aflatoxin internal standards. To address this challenge, an Agilent ZORBAX Eclipse Plus C18, 2.1 x 100 mm column with 1.8 µm particles was used to deliver the necessary peak resolution. The ability of the Agilent 1290 UHPLC to easily deliver flow against a back pressure of over 700 bar proved to be crucial.
Maximum allowable mycotoxin levels in cereals and fruit range from 10 to 4,000 µg/kg, but levels in baby food are much lower – well below 1 µg/kg for aflatoxins and ochratoxin. The method delivered the required sensitivity.
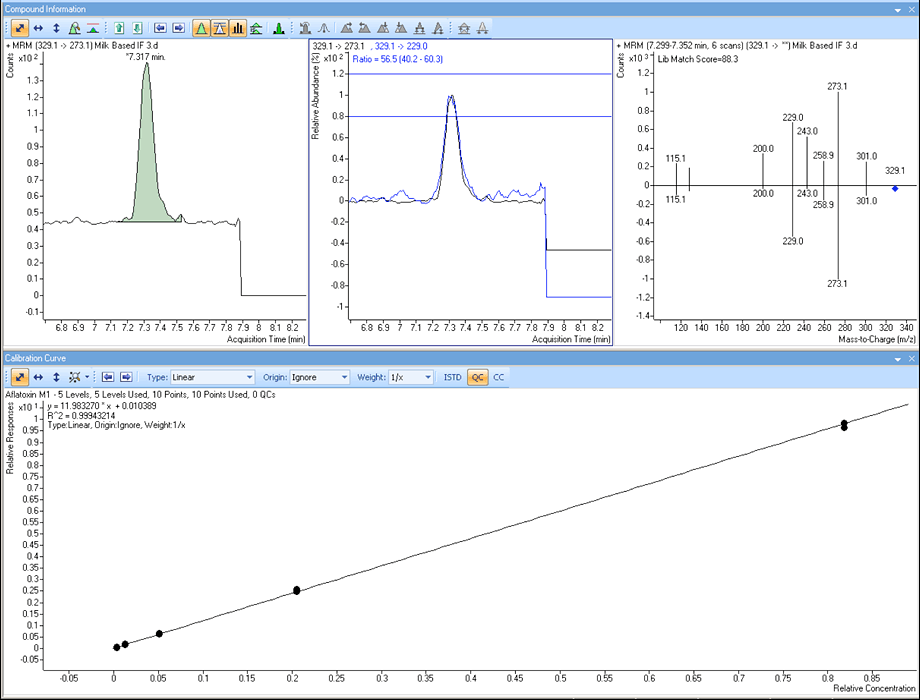
 Enlarge
Enlarge
Figure 2. Agilent LC/MS/MS easily quantitates and identifies aflatoxin M1 in infant formula below the EU limit with a triggered MRM “spectrum” match that had eight transitions even at this very low level.
Multi-mycotoxin screening in infant formula
For this analysis, Dan Hengst and Katerina Mastovska from Covance Laboratories’ Nutritional Chemistry and Food Safety division (Madison, WI) utilized the same Agilent instrument setup as the previous example. The same suite of compounds was also analyzed, but used a longer 150 mm column. They achieved effective separation and quantitation of sub-ppb mycotoxins in infant formula in under 10 minutes, with no need for concentration. The use of labeled internal standards allowed for a method run against a simple solvent calibration.
Regulators also require determination of aflatoxin M1 in food of animal origin. In infant formula, for example, the maximum level is set to very low levels – down to 0.025 µg/kg in the EU. It was possible to also add this compound to the method and to achieve the neceassary sensitvity.
Additionally, a library of triggered MRM spectra verified eluting peaks and increased confidence in peak identification. Collection of spectra through the MRM function was much faster than other ion-trap-based product ion scans and did not compromise the prime objective of monitorring and measuring eluting peaks. Triggered MRM also had high sensitivity, allowing verification even at very low levels. For these reasons, it was concluded that Triggered MRM should be used as part of a routine quantitative monitoring. (Figure 2).
 Enlarge
Enlarge
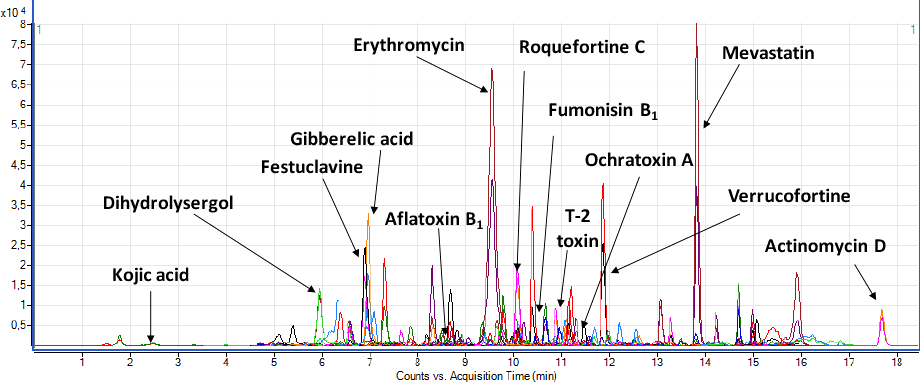
Figure 3. Screening multiple mycotoxins in hazelnut.
Broad scope screening of mycotoxins in nuts
There is also interest in screening for a much wider array of mycotoxins without necessarily needing to quantitate all of them. Such activities allow emerging threats to be detected and new compounds of concern to get further focus. The Tulln research team had access to approximately 400 mycotoxins – enabling them to populate the Agilent Triple Quadrupole LC/MS method with optimal transitions and retention times – developed for screening nuts. The objective was to develop a metrget="_blank">develop a metnot typically have access to many of these compounds – allowing researchers to search for them if using the same method and instrument setup. Figure 3 shows a screen of a naturally contaminated hazelnut that contained aflatoxins, alternaria toxins, mycophenolic acid, and T-2 toxin – found for the first time in hazelnuts.
Agilent offers a spectrum of mycotoxin testing solutions for many applications
The use of Agilent LC/MS solutions for multi-mycotoxin assays extends to other food matrices. Even high fat matrices like sesame or peanut butter have been similarly covered. In upcoming articles, we will further explore broad scope screening by LC/MS with extended applications. Until then, why not take a few moments to learn more about Agilent’s food testing solutions.
>> Update My Profile | Subscribe to Access Agilent | Article Directory
Figure 1.
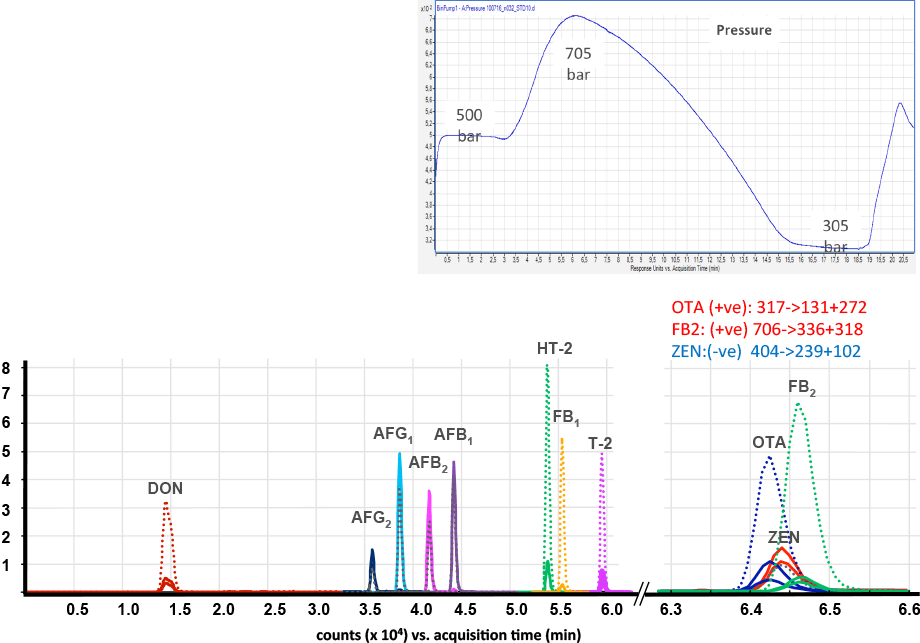
Coelution of target mycotoxins and their internal standards – dotted lines are labeled compounds and solid lines are the native (or target) compounds.