Access Agilent eNewsletter June 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Fast Quality by Design method development and transfer from UHPLC to HPLC
By Andreas Tei
Agilent Pharma Segment Manager – Small Molecules
and Vinayak Azakaprakalam
Agilent Application Chemist
Nobody wants an analytical method to fail in a pharmaceutical QA/QC lab. To reduce the risk of such failures, the Quality by Design (QbD) approach in method development uses design of experiments (DoE) and multivariate analysis to develop a design space. The design space defines the experimental conditions in which changes to method parameters will not significantly affect the results. Agilent tools accelerate this approach, reducing method failures and out-of-specification results.
In the pharmaceutical industry, method development, validation, and transfer to QA/QC laboratories are routine. However, it is time-consuming to do column screening and method development using conventional HPLC columns under QbD principles. Efficiency increases dramatically when you use UHPLC methods on short, sub-2-µm columns during the screening phase.
Method transfer from UHPLC to HPLC conditions without compromise of the critical method attributes (CMAs) – such as the active pharmaceutical ingredient (API) resolution and peak purity – can be a challenge. Agilent Method Translator and Agilent Intelligent System Emulation Technology (ISET), combined with Fusion QbD Method Development and Validation Software, provide fast and efficient method transfer from UHPLC to HPLC within the QbD approach.
 Enlarge
Enlarge
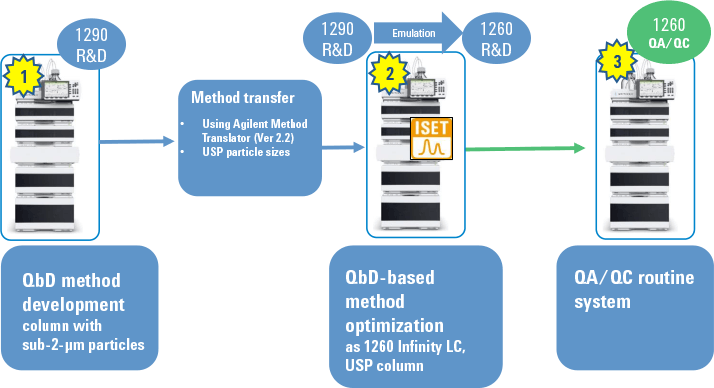
Figure 1. Overall procedure for successful transfer of an optimized QbD method from UHPLC to HPLC.
 Enlarge
Enlarge
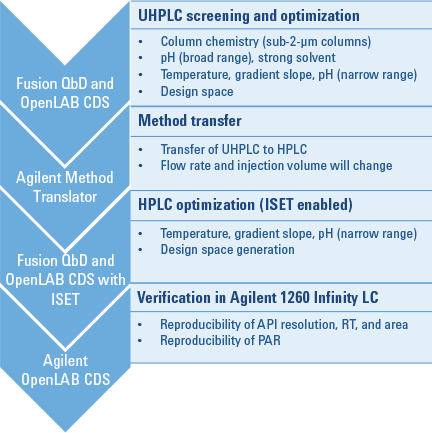
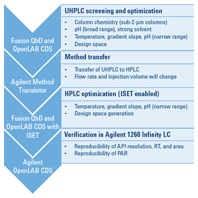
Figure 2. QbD workflow, showing software packages on the left and detailed steps on the right.
An outline for successful creation of a robust method
We recently demonstrated this efficient method transfer. In the first step, we created the method using QbD concepts on an Agilent 1290 Infinity Binary LC with a sub-2-µm column. We then used the Agilent Method Translator to calculate method conditions for the change from the UHPLC sub-2-µm column to an HPLC column with larger particles. We executed this step on an Agilent 1290 Infinity LC emulated as an Agilent 1260 Infinity Binary LC, which represented the target system. We then optimized the method to create a new design space for the target system. Figure 1 summarizes the process.
Accelerated method development in more detail
We began the workflow by screening six short sub-2-µm columns, two organic solvents, and five different aqueous solvents of varying pH. For this step, we used an Agilent 1200 Infinity Series Method Development Solution and Fusion QbD Software (Figure 2). The best conditions were provided by an Agilent ZORBAX Rapid Resolution High Definition (RRHD) Eclipse Plus C8 column, at pH 7 with a gradient time of 10 minutes. We then further optimized these conditions to achieve a robust method. A design space was then generated for the UHPLC method.
The Agilent Method Translator helped us to transfer the newly created UHPLC method to an HPLC method. To optimize the transferred method by DoE, we varied temperature, gradient slope, and pH to ensure the method’s robustness. We compared the resulting HPLC design space with the previously generated UHPLC design space, to verify the proven acceptable range (PAR) within the design space on both systems. Finally, we verified the HPLC method for reproducibility of API area, retention time, and resolution on the target system.
Table 1 shows the time needed for each step of the workflow. The table records the time to run sequences, prepare samples and solvents, and configure instruments. It shows that screening with short sub-2-µm columns is much less time-consuming than screening with conventional columns. With this approach, we reduced the method development time by two working days.
Workflow step |
Duration (days) using sub-2 µm columns |
Workflow step |
Duration (days) using conventional columns |
|---|---|---|---|
UHPLC screening |
2 |
HPLC screening |
5 |
UHPLC optimization |
1 |
|
|
HPLC optimization |
2 |
HPLC optimization |
2 |
Verification on Agilent 1260 Infinity LC |
1.5 |
Verification on Agilent 1260 Infinity LC |
1.5 |
Total |
6.5 |
Total |
8.5 |
Table 1. Time required for each step of a QbD workflow reveals that you can save two days by using sub-2-µm columns in the screening step.
 Enlarge
Enlarge
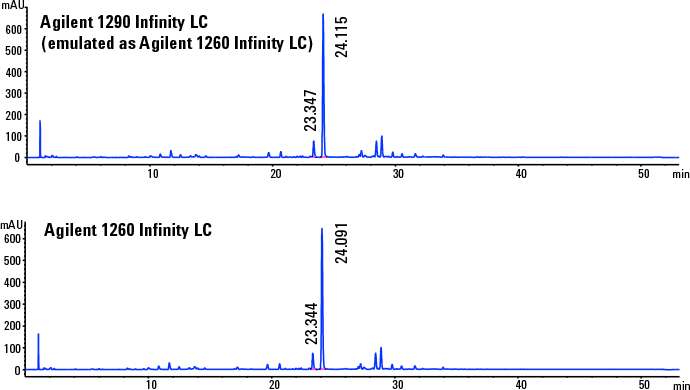
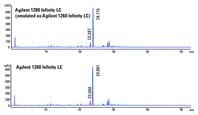
Figure 3. Chromatograms demonstrate successful transfertween the two LC systems.
Verification of consistency between emulation and target
The newly developed HPLC method on the Agilent 1290 Infinity LC system in emulation mode was replicated on the target Agilent 1260 Infinity LC. The difference of retention times and resolution of the API between the two chromatograms was within the acceptance limits (Figure 3). We verified method reproducibility with six replicates. The results showed that RSDs of API areas, retention times, and resolution were within the acceptance limits. For example, the percent deviation of API resolution was 4.2 and retention time was 0.1. In addition, we replicated PAR on the Agilent 1260 Infinity LC to confirm that we met the CMA performance requirements (data not shown).
Full details of this work are freely available in Agilent publication 5991-5701EN. To learn more about Agilent solutions for QbD implementation in pharmaceutical development, download the informative primer 5991-2166EN today.
>> Update My Profile | Subscribe to Access Agilent | Article Directory