Access Agilent eNewsletter June 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Ask the Expert: How do I analyze biosimilars with reversed-phase liquid chromatography?
By Koen Sandra
Research Institute for Chromatography, Kortrijk, Belgium
and Maureen Joseph
Agilent Biopharma Applications
In last month’s article on biosimilars, we answered some questions on the first steps in biosimilar characterization using affinity chromatography. This powerful technique is most commonly used as a first stage in the purification of recombinant proteins and in the selection of promising clones. This month we turn our attention to reversed-phase LC, to examine intact structures and produce peptide maps and, when coupled to MS, to increase confidence in the results.
Q. What is reversed-phase LC?
Answer: Reversed-phase LC is a powerful tool for characterizing mAbs and biosimilars. It is used with UV detectors in QC monitoring and product release testing, and with MS to characterize everything from an intact antibody down to peptide fragments in a digested antibody.
 Enlarge
Enlarge
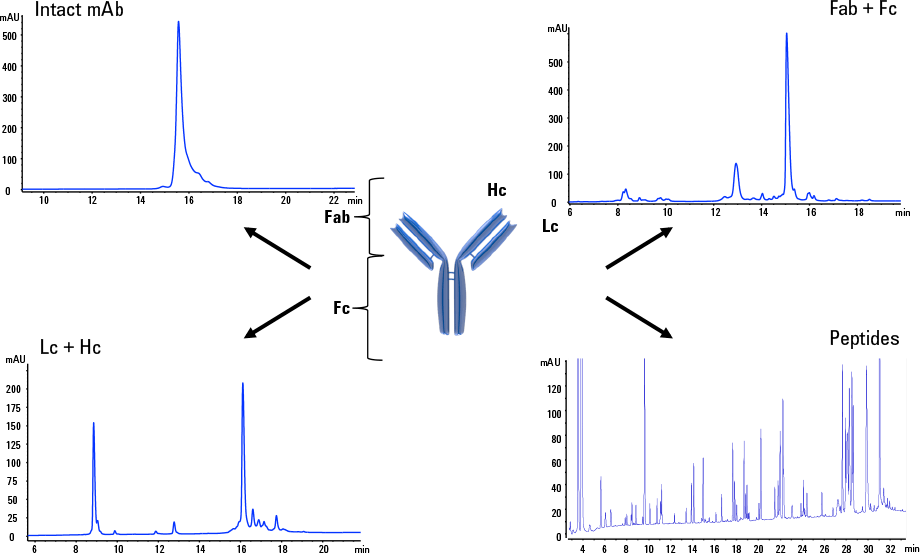
Figure 1. Four examples of reversed-phase LC analysis used to characterize mAbs and biosimilars.
 Enlarge
Enlarge
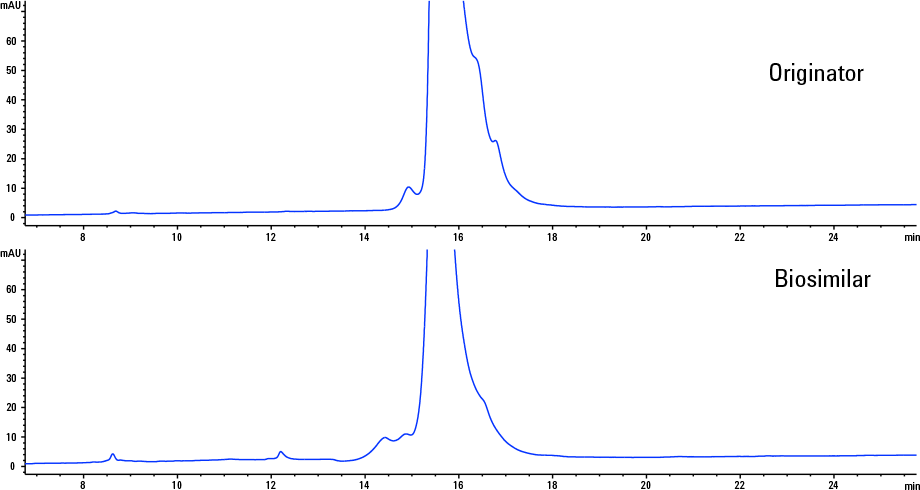
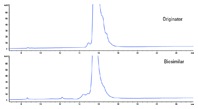
Figure 2. Reversed-phase LC gradient separation of intact Herceptin originator and a biosimilar on an Agilent ZORBAX RRHD 300SB-C8, 2.1 x 100 mm, 1.8 µm column.
 Enlarge
Enlarge
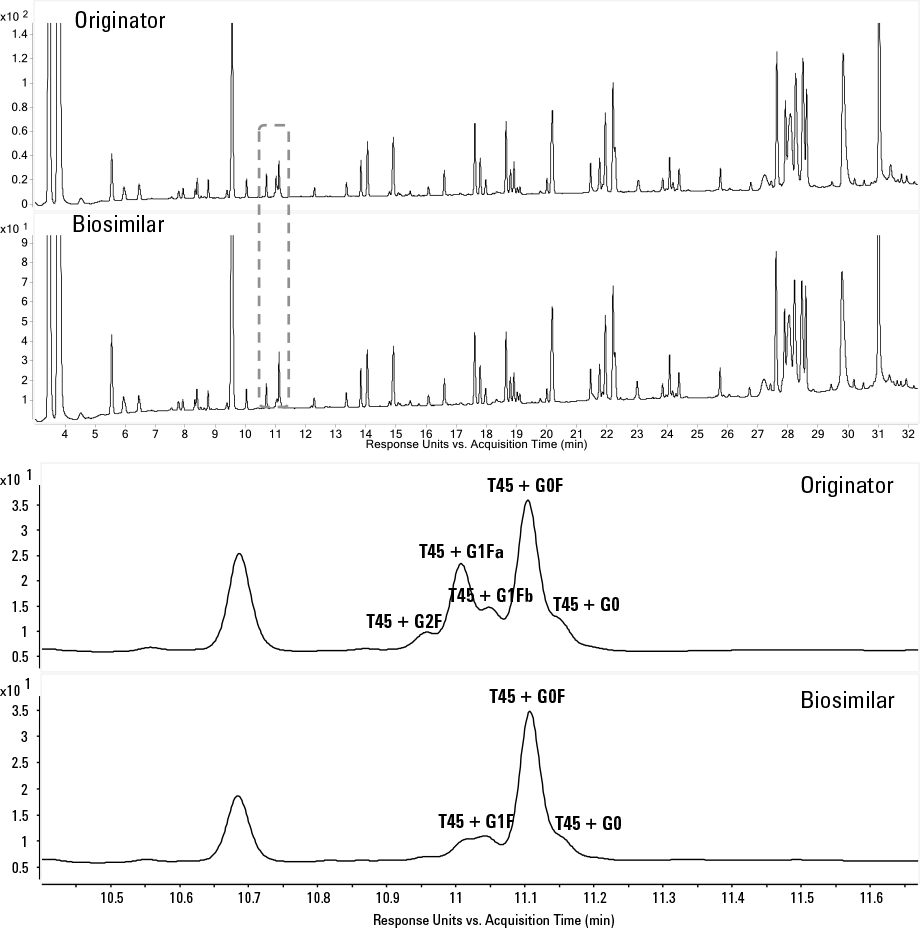
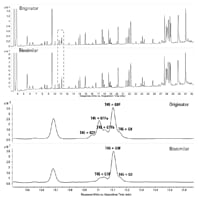
Figure 3. Reversed-phase LC separation of tryptic peptides of Herceptin and a biosimilar on an Agilent AdvanceBio Peptide mapping column, 2.1 x 100 mm, 2.7 µm column.
Q. Why use reversed-phase LC to analyze biosimilars?
Answer: Reversed-phase columns with smaller particle sizes and superficially porous particles provide extra resolution and save time when characterizing mAbs and biosimilars. Figure 1 shows four examples of RP separations of intact, heavy chain (Hc)/light chain (Lc), fragment antigen-binding (Fab/Fc), and tryptic peptides of Herceptin. LC and LC/MS were used together with UHPLC columns, packed with sub 2 µm fully porous particles, or HPLC columns packed with 2.7 µm superficially porous particles. The goal in all these separations was to determine identity and purity, so that innovator and biosimilar therapeutics could be characterized and compared in the shortest possible time. Note that intact mAb, Fab/Fc, and Lc/Hc can all be separated using exactly the same chromatographic method.
Q: What about using reversed-phase LC for examination of intact mAbs?
Answer: When it comes to separating an intact mAb with a typical RP gradient, minor changes in the organic solvent substantially affect the separation. We need a shallow gradient (low % B change/min) over a narrow total organic range. Figure 2 shows an example of the separations of a Herceptin originator and biosimilar. The biosimilar was purified on the Agilent Bio-Monolith Protein A column as shown in last month’s article. Several peaks are identifiable in the UV chromatogram due to the high resolution provided by the Agilent ZORBAX RRHD 300SB-C8, 1.8 µm column fitted to an Agilent 1290 Infinity LC.
Q: Can reversed-phase LC help me understand minor modifications?
Answer: Yes, it can. More information becomes available when separating fragments of mAbs including Fab/Fc, Lc/Hc, and peptides. Figure 3 shows the RPLC-UV separation of the tryptic peptides of Herceptin and a biosimilar on the Agilent AdvanceBio Peptide Mapping column fitted to an Agilent 1290 Infinity LC. Peptide mapping can provide an enormous amount of detail on the primary structure and post-translational modifications, allowing extensive characterization and comparison between an originator and biosimilar. While the peptide maps shown in Figure 3 are highly comparable, differences in glycosylation are revealed. The different glycosylated variants were nicely resolved chromatographically and observed by UV detection. Undergalactosylation of the biosimilar became apparent. Several other impurities identified from the peptide mapping data included lysine truncation, methionine oxidation, and asparagine deamidation. All of these modifications were minor and in low quantities, but could be critical to potency and safety of a mAb.
These examples of reversed phase LC of Herceptin and a biosimilar are key separations for characterizing and comparing mAbs. In the next issue of Access Agilent, we will look at charge variant and aggregate analysis, wrapping up the series with an article on the powerful software tools you can use to speed up your analyses. All the articles are freely available in detail in Prepping Biosimilars.
Agilent biopharma and LC solutions
High resolution, optimized gradient LC methods with sub-2 µm and superficially porous columns, combined with UV and accurate mass Q-TOF, provide the insights you need for your biosimilars. These advanced methods help you correctly determine molecular weight and identity, confirm purity, and find difficult to resolve impurities. Learn more about Agilent biopharma solutions to help advance your biopharma research.
Additionally, Agilent offers a broad range of liquid chromatography products and solutions Tens of thousands of laboratories worldwide rely on Agilent LC Systems to provide the high quality results they need, run after run, day after day. Over one million LC modules are deployed around the globe, delivering unparalleled performance, productivity, and reliability. Contact an Agilent Representative today to find the right LC solution for your applications.
>> Update My Profile | Subscribe to Access Agilent | Article Directory