Access Agilent eNewsletter June 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Quick, easy, accurate residual solvents analysis with Agilent 7697A Headspace Sampler
By Cassie Eisele
Agilent Product Manager
Pharmaceutical quality assurance (QA) labs are required to use regulated methods to analyze for residual solvents left over from the manufacturing of active pharmaceutical ingredients (APIs) or final products. These regulations include United States Pharmacopeia (USP) <467>, ICH Q3C and European Pharmacopeia (EP) general chapter 5.4 – and vary regionally, depending on where the drugs are sold. All drug substances, excipients, and products must be monitored. While residual solvents can have an effect on crystalline form, solubility, bioavailability, and stability, the most important consideration of this analysis is the safety and environmental hazards associated with them.
When patient safety is at stake there is no room for error. Laboratories have to have complete confidence that their data is accurate and reliable. Comprehensive testing solutions, such as those from Agilent – including the 7697A Headspace Sampler, Residual Solvents Analyzers, and Method Conversion Wizard – can deliver the accuracy needed to provide busy lab technicians with the confident results they require. In this article we will examine the results from a residual solvents analysis and show how using the right testing solutions can provide your lab with quick, easy deployment when it’s time for an upgrade.
 Enlarge
Enlarge
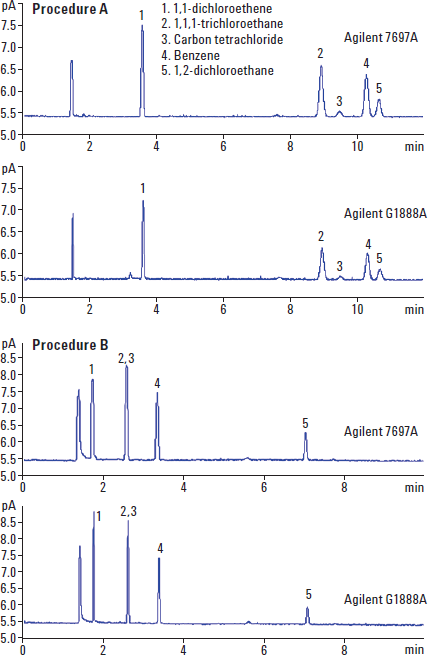
Figure 1. Comparative chromatograms of Procedure A and Procedure B for the Agilent 7697A and Agilent G1888A Headspace Samplers, at USP limit concentrations.
Increase testing precision by controlling ambient pressure fluctuations
Previous generation headspace samplers, such as the Agilent G1888A Headspace Sampler, were designed to vent the pressurized vial to ambient pressure while filling the sample loop. The Agilent 7697A Headspace Sampler introduced the use of onboard electronic pneumatics control to allow the headspace to control the final vial pressure seen in the sample loop during the loop filling process. Controlling the final vial pressure and adding in barometric pressure compensation removes any effects from ambient atmospheric pressure fluctuations in the lab. This means your lab will get increased precision in its day-to-day runs and your technicians will get the same results every time. It can also allow your techs to get better sensitivity from the instrument since they are able to get more sample on the GC column. To help you optimize these vial pressurization parameters for your headspace, you can refer to Application Note 5990-9106EN [1].
When your lab is upgrading to a newer instrument, it can sometimes be difficult to implement your old methods on the new instrument. In a previous Access Agilent article we discussed using the Method Conversion Wizard to migrate your older headspace method to the 7697A Headspace Sampler. For more information on implementing your older G1888A or 7694 Headspace Sampler residual solvents methods on the 7697A Headspace Sampler, you can refer to the new Agilent Technical Overview on instrument migration, 5991-5182EN [2].
Through comparative studies we were able to demonstrate that not only does the 7697A Headspace Sampler meet the USP <467> requirements, but that it also gives similar or better results than the G1888A Headspace Sampler for system suitability (Figure 1).
In addition, we also did comparative studies to show how the Agilent 7697A Headspace Sampler, with its inert sample pathway, thermal zone stability, and onboard electronic pneumatics performs similarly or better than the G1888A Headspace Sampler in regards to precision and accuracy. In general, we were able to show that the Agilent 7697A Headspace Sampler offers an improvement in area count precision (Table 1).
Peak no. |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13,14 |
15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Agilent 7697A |
1.54 |
0.48 |
1.65 |
1.86 |
1.71 |
0.79 |
1.63 |
1.29 |
1.64 |
1.66 |
1.44 |
1.39 |
1.33 |
1.27 |
Agilent G1888A |
1.33 |
6.72 |
1.90 |
2.00 |
2.00 |
1.21 |
1.94 |
1.84 |
1.48 |
2.01 |
2.02 |
1.97 |
1.99 |
2.02 |
Table 1. % RSD comparison of Agilent 7697A and Agilent G1888A Headspace Samplers. RSD is for seven injections.
This detailed comparison of the Agilent 7697A Headspace Sampler and the G1888A Headspace Sampler provides a resource for method transfer and S.O.P. documentation for laboratories replacing their older Agilent headspace with a 7697A Headspace Sampler. It also gives recommended method parameters for migrating a method from the G1888A Headspace Sampler to get you started on your method development.
Agilent Analyzers provide quick ramp-up
If your lab is just starting out with residual solvents analysis or upgrading to a new instrument, you need an analysis solution that will get you up and running quickly and with minimal disruption to your operations. Agilent offers three different Residual Solvents Analyzers that will enable your lab to be validated and running samples with minimal start-up time. All three analyzers come pre-configured per industry standards and chemically tested to ensure optimal analysis of Class 1 and Class 2A/B solvents. Depending on your lab’s requirements for sample identification – or both identification and additional confirmation of organic contaminants – choose from Agilent’s GC Analyzers with either a single or dual FID setup. Your lab can also consider Agilent’s GC/FID/MS analyzer to achieve both identification and additional confirmation.
All of these analyzers come with the inert sample path and thermal zone stability of the Agilent 7697A Headspace Sampler combined with the Agilent 7890B GC to exceed USP <467> requirements. To aid in fast ramp-up, the solution also includes all necessary parts, consumables, and a quick start guide for method validation.
Everything you need for robust and repeatable analysis
Agilent offers a wide array of Gas Chromatography (GC) and Mass Spectrometry (MS) solutions that offer increased accuracy, more confident results, higher throughputs, and operational efficiencies. To see how these solutions can increase efficiencies in your busy lab, contact your Agilent Representative today.
References
- R. L. Firor “Optimizing Vial Pressurization Parameters for the Analysis of USP <467> Residual Solvents Using the 7697A Headspace Sampler” Agilent Technologies, publication number 5990-9106EN.
- “System Parameter and Performance Comparison Between Agilent 7697A and Agilent G1888A Headspace Samplers for USP <467>” Agilent Technologies, publication number 5991-5182EN.
>> Update My Profile | Subscribe to Access Agilent | Article Directory