Access Agilent eNewsletter February 2015
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Agilent solutions for continuous, real-time air sampling and analysis
By Craig Marvin
Agilent Global Environmental Industry Manager
The presence of volatile hydrocarbons in urban air can contribute to the formation of ground-level ozone, one of the main ingredients in urban smog. Compounds of interest range in volatility from acetylene (ethyne) to trimethylbenzene. They are generally known as "ozone precursors." Vehicle emissions are the main source of these compounds.
European and U.S. regulations require round-the-clock monitoring of hydrocarbons in all major urban centers to establish and monitor the link between periods of high traffic density and high pollution levels of key compounds, such as benzene, toluene, xylene, and buta-1,3-diene. Continuous, real-time monitoring provides an insight into levels of vehicle emissions and is used to monitor emission fluctuations caused by weather conditions, such as wind direction, precipitation, and temperature inversion.
Cryogen-free thermal desorption technologies were developed in response to this increasing demand for measurement of ambient air toxins and ozone precursors. These offer an automated, method-compliant analytical platform for both canisters and sorbent tubes.
In this article, we focus on a couple of cryogen-free approaches that take advantage of the capabilities offered by Agilent and Markes air monitoring and analysis systems.
Unattended operation, round-the-clock monitoring
For our first example, we validated a system for on-line monitoring of 27 ozone precursors specified by European regulators, with the addition of 1,2,4-trimethylbenzene, 1,3,5-trimethylbenzene, and isoprene (present in the gas standard employed). Key factors included the need for a system that could operate unattended and round-the-clock – so cryogen-free operation was essential. The system also needed to collect samples every hour with as much of the hour as possible dedicated to sampling. Finally, detection limits had to be below 0.5 ppb (ideally 0.1 ppb).
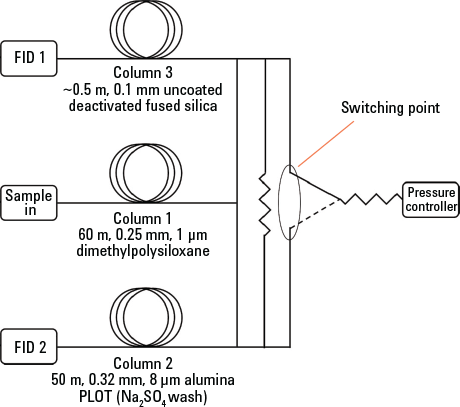
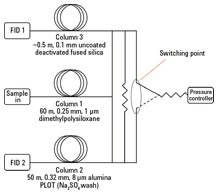
For better separation of C4 and C5 components, a two-column system with a Deans’ switch was used (Figure 1). All the effluent from the focusing trap of the desorber passes through the dimethylpolysiloxane column at 40 °C. The C2 to C5 compounds were not resolved on this column at this temperature, and therefore passed via the Deans’ switch, into the alumina PLOT column as they elute from the primary column. For the first 17.5 minutes of the run, the Deans’ switch between the primary and secondary columns worked in this direction.
At this point, all the compounds eluting from the primary column were C6 and higher, and were well resolved. Activation of the Deans’ switch directs effluent from the primary column to the deactivated, uncoated fused silica link and to the other FID. Figure 2 shows a plot for the calibration gas run through this two-column system. Read full details of these experiments in the free Agilent publication 5991-2823EN.
 Enlarge
Enlarge
Figure 1. Schematic representation of the two-column ozone precursor analytical system.
 Enlarge
Enlarge
Figure 2. Chromatograms for a ppb-level 30-component calibration standard (generated by the National Physical Laboratory, UK) on the two-column analytical system. All components had a concentration from 3.83 to 4.16 ppb, with uncertainties for each of ±0.08 ppb.
 Enlarge
Enlarge
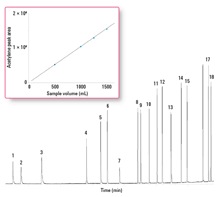
Figure 3. Splitless analysis of the C2 to C6 portion of an ozone precursor standard (2 to 10 ng of components on-column) using an alumina PLOT column.
Effective analysis of volatile, small molecule hydrocarbons
Of all the ozone precursors, the C2 hydrocarbons present a particular challenge due to their extremely high volatility and small molecular size. Of these, acetylene (b. p. -89 °C), is the most difficult to trap. The Markes CIA Advantage trapping system is adept at handling compounds as volatile as this without liquid cryogen coolant, due to the uniquely powerful combination of trap dimensions, sorbent capacity, and electrical cooling. Figure 3 shows an example of the data generated by the system, again using a Deans’ switch technique with alumina and PLOT GC columns. Even though splitless injection was used for maximum sensitivity, the peak shape of the early-eluting compounds remained good. The inset shows quantitative cryogen-free retention of acetylene from air volumes up to 1.5 L. Read more on this approach in Agilent publication 5991-2842EN.
Quick, easy, uniform air sample collection with Agilent and Markes solutions
Analysts need to quickly and easily gather uniform samples and execute multiple testing methods to detect an increasing list of harmful air toxins. Agilent’s high quality GC/MSD portfolio – consisting of Agilent J&W GC columns, 7890B GC and the 5977A Series GC/MSD, along with the Markes Unity Thermal Desorber – provides a complete air analysis solution to meet these challenges.
Acknowledgment
We thank Markes International Ltd for use of chromatograms and supporting text.
>> Update My Profile | Subscribe to Access Agilent | Article Directory
Figure 2.
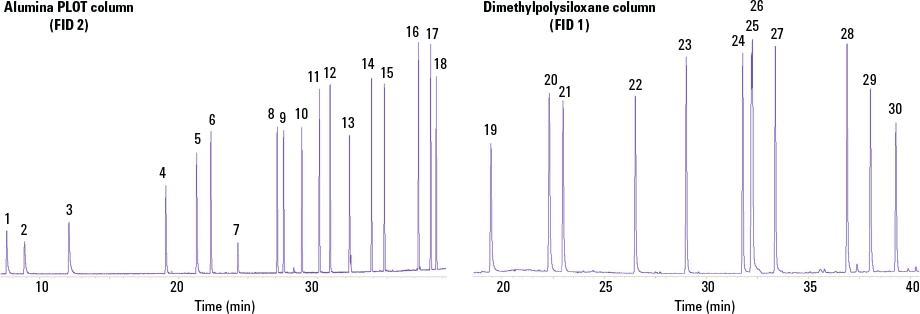
Peak ID
- Ethane
- Ethene
- Propane
- Propene
- 2-Methylpropane
- n-Butane
- Acetylene
- trans-But-2-ene
- But-1-ene
- cis-But-2-ene
- 2-Methylbutane
- Pentane
- Buta-1,3-diene
- trans-Pent-2-ene
- Pent-1-ene
- 2-Methylpentane
- Isoprene
- n-Hexane
- Benzene
- 2,2,4-Trimethylpentane
- n-Heptane
- Toluene
- Octane
- Ethylbenzene
- m- and p-Xylene
- m- and p-Xylene
- o-Xylene
- 1,3,5-Trimethylbenzene
- 1,2,4-Trimethylbenzene
- 1,2,3-Trimethylbenzene
Chromatograms for a ppb-level 30-component calibration standard (generated by the National Physical Laboratory, UK) on the two-column analytical system. All components had a concentration from 3.83 to 4.16 ppb, with uncertainties for each of ±0.08 ppb.
Figure 3.
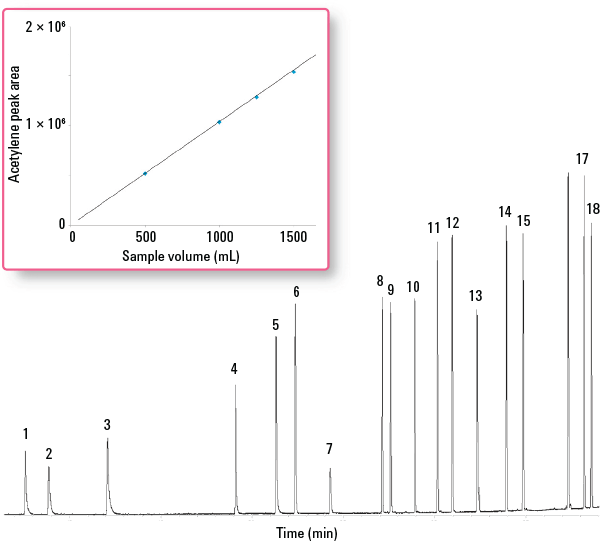
Peak ID
- Ethane
- Ethene
- Propane
- Propene
- 2-Methylpropane
- n-Butane
- Acetylene
- trans-But-2-ene
- But-1-ene
- cis-But-2-ene
- 2-Methylbutane
- Pentane
- Buta-1,3-diene
- trans-Pent-2-ene
- Pent-1-ene
- 2-Methylpentane
- Isoprene
- n-Hexane
Splitless analysis of the C2 to C6 portion of an ozone precursor standard (2 to 10 ng of components on-column) using an alumina PLOT column.