Access Agilent eNewsletter, March 2014
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Robust and reproducible determination of monoclonal antibody titers
By Phu T. Duong
Agilent Biocolumns Applications Chemist
Expression of monoclonal antibody (MAb) titers – and capture from a cell culture broth – demands speed, quantitative accuracy, and precise determination during process development. Agilent Bio-Monolith Protein A affinity columns deliver fast, accurate recovery of MAb titers over a broad range of concentrations. Designed for the analytical separation of all human and mouse immunoglobulin G (IgG) except for class 3, Agilent Bio-Monolith Protein A affinity columns help you perform cell culture optimization. Their unique monolithic format gives you fast separations, high flow rates, improved quantitative accuracy, and long column life.
 Enlarge
Enlarge
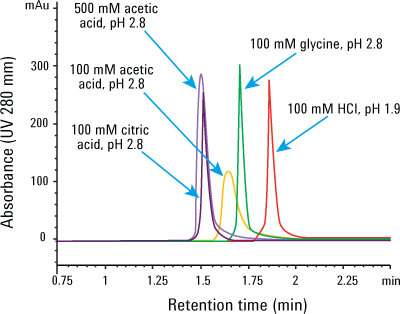
Figure 1. Many choices: influence of elution buffer on IgG1 peak height and shape. Injection: 4 µL of 1.25 mg/mL IgG1 mixed with 20 mg/mL Escherichia coli proteins.
In the following examples, we show how robustness and reproducibility deliver long column life with minimal clogging, even with complex samples. As a result, you do not need to clean or replace the column as often as you expect.
Polymeric particles perform well for acidic pH
Figure 1 shows the influence of the elution buffers on the Agilent Bio-Monolith Protein A column. The data clearly indicate that you can elute the IgG1 peak with many different acidic buffers, even down to pH 1.9 with HCl, because of the column’s robust polymeric methacrylate packing. Glycine, citric acid, and acetic acid at pH 2.8 are also effective buffers. These eluents provide very comparable peak height for IgG1, with variable retention times. Peak shapes and peak widths are very similar, except with 100 mM acetic acid. However, this elution buffer, when increased to 500 mM, gained enough strength to make peak height and shape comparable to that of the other elution buffers.
Minimal clogging, minimal cleaning
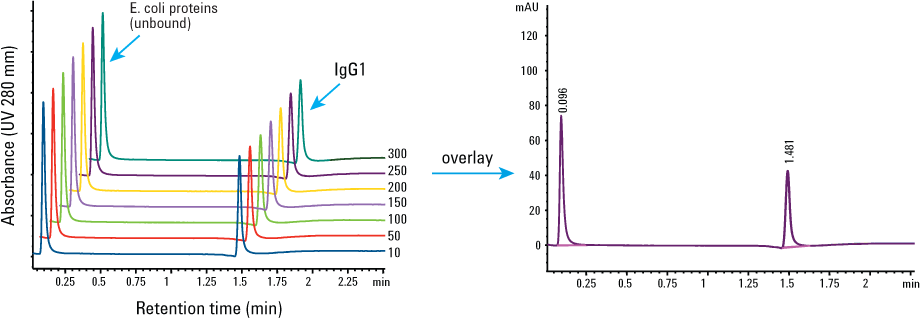
We assessed the column reproducibility over 300 consecutive injections, with the results shown in Figure 2 and Table 1. For every 20 injections, we used three injections without sample as a washing step to help elute as much residue from the column as possible. You can see that the retention time, peak area, and peak width remained the same. The column did not lose its binding and separating capacity for IgG1, and clean-in-place was not required at this point.
 Enlarge
Enlarge
Figure 2. Reproducibility of the Agilent Bio-Monolith Protein A column, 300 injections before clean-in-place. Injection: 1 µL of 1.25 mg/mL IgG1 mixed with 10 mg/mL E. coli proteins.
Injection number |
IgG1 retention time (min) |
IgG1 area (mAu/s) |
IgG1 peak width (min) |
|---|---|---|---|
10 |
1.48 |
89 |
0.2 |
50 |
1.48 |
88 |
0.2 |
100 |
1.48 |
88 |
0.2 |
150 |
1.48 |
87 |
0.2 |
200 |
1.48 |
88 |
0.2 |
300 |
1.48 |
87 |
0.2 |
Table 1. Retention time, peak area, and peak width of IgG1 from 300 injections before clean-in-place. Standard deviation of n = 300 was 0.75.
Signs that your column must be cleaned include increased backpressure, peak splitting, shorter peak height, and reduced binding/absorbing of materials. It is best to clean the column before these signs appear (typically after 20 to 50 runs), even though the Agilent Bio-Monolith Protein A column operates successfully over many more runs.
Full details of these investigations are in our latest Bio-Monolith Protein A Application Note (5991-2990EN).
Reproducible affinity chromatography
Agilent Bio-Monolith Protein A columns deliver the high capacity and performance you need for reproducible affinity chromatography. Bio-Monolith Protein A affinity columns offer all the advantages of a specially designed, continuous short polymeric bed. These inherent features enable highly reproducible separation and quantitation of IgG from cell culture supernatants. Separations are independent of flow rate, with no diffusion, no pores, and no void volume. Transport between mobile and stationary phase is very rapid to speed up method development time and decrease costs.
These columns are just part of the Agilent bioseparation family, which includes Agilent biocolumns for consistent, exceptional performance to separate and characterize peptides and proteins. Explore and learn more about these columns today.
>> Update My Profile | Subscribe to Access Agilent | Article Directory