Access Agilent eNewsletter, April 2014
>> Update My Profile | Subscribe to Access Agilent | Article Directory

Better ion-exchange chromatography for biomolecules
By Linda Lloyd
Agilent Product Manager, Biocolumns
Ion-exchange (IEX) is widely used for separating biomolecules based on differences in ionic charge. It is a mild, non-denaturing technique that does not require organic solvents and is therefore frequently used to characterize proteins in their native or active form, and for purification. We discuss here some of the main points to consider when developing methods for IEX. You’ll find these and more in the upcoming Agilent Ion-Exchange Chromatography for Biomolecule Analysis: a “How to” Guide.
 Enlarge
Enlarge
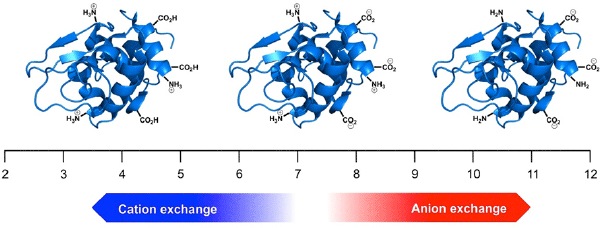
Figure 1. The effect of pH on net protein charge.
Develop an effective ion-exchange method
The technique of ion-exchange is suitable for separating proteins with differing isoelectric points, but it is equally valuable in separating charged isoforms of a single protein. However, it is important to remember that proteins such as monoclonal antibodies are incredibly complex. A typical mAb comprises over 1,300 individual amino acids. Of these, perhaps 130 have acidic side chains and 180 have basic residues. The likelihood is that a monoclonal antibody will have a net positive charge at neutral pH and therefore should be separated using a cation-exchange column (Figure 1). However, it is difficult to predict the actual isoelectric point, pI, of such a molecule, and so some method development or optimization should be anticipated.
Sample preparation
Sample preparation for IEX is not unlike that for any protein analysis. The most important aspect is that the sample must be soluble in the eluent and should ideally be dissolved in the mobile phase itself. To protect the column from possible damage, samples should be filtered before use to remove particulates, but filtration should not be used to compensate for poor sample solubility – an alternative eluent may need to be found. In addition, samples should be made up fresh and analyzed as soon as possible. Refrigeration can increase the shelf life of samples and delay bacterial growth that can otherwise develop quickly in buffer solutions.
Choosing column media
There are a range of IEX columns available. With ion-exchange, the first consideration should be “anion or cation-exchange?” There is also the choice of strong or weak ion-exchange. In most circumstances it is best to start with a strong ion-exchange column, followed by weak ion-exchangers to provide a difference in selectivity if necessary. The choice between anion- and cation-exchange depends on the isoelectric point of the protein(s) of interest.
 Enlarge
Enlarge
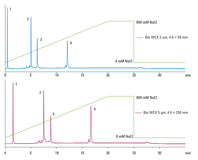
Figure 2. Protein separation on Agilent Bio WCX columns. Faster analysis times were achieved with smaller particle size and shorter column length – samples eluted from the longer column in 17 minutes but in only 12 minutes from the shorter column.
 Enlarge
Enlarge
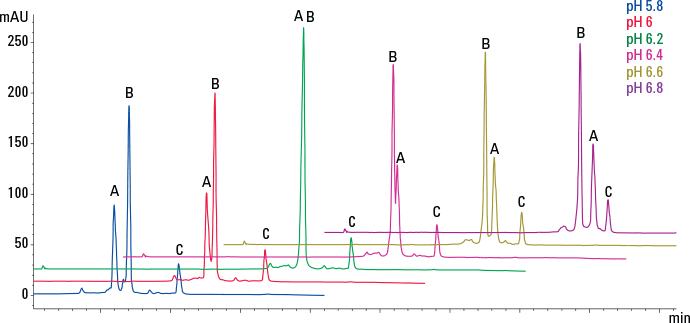
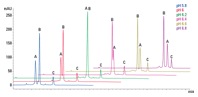
Figure 3. pH scouting separates a three-protein mix using dynamically mixed quaternary gradients.
Choosing columns
Biomolecules must be able to freely permeate the particles. Non-porous spherical particles provide the highest resolution for analytical separations, where column loading capacity is not a major concern. When considering particle size, use smaller particles for higher resolution, though this results in higher back pressure. Shorter, 50 mm columns can be used for more rapid separations, particularly with smaller particles, and longer 250 mm columns for additional resolution (Figure 2). Smaller id columns reduce solvent consumption, and smaller injection volumes are beneficial with limited samples.
Flow rate
Typical flow rate for 4.6 mm id columns is 0.5 to 1.0 mL/min, but for some applications the speed of analysis is crucial. Shorter columns can be used to reduce the analysis time – 50 mm instead of the conventional 150 mm or 250 mm – or flow rates can be increased, or both (taking care not to exceed column pressure limitations).
Mobile phase
The mobile phase should contain buffer to maintain the desired operating pH, typically 20 mm. The pH and ionic strength of the buffer can affect resolution on weak ion-exchange products and so the optimum conditions should be found experimentally (Figure 3). Addition of NaCl to the mobile phase will alter the pH, so re-adjust as necessary.
Column conditioning and equilibration
Column equilibration and cleanup phases of the gradient are critically reproducible for IEX. Protein elution is achieved by increasing the ionic strength or changing the eluent pH, or both, and so at the end of each analysis the column must be equilibrated back to the starting conditions, ionic strength, and pH. If not, the next column run will have a different profile as the protein will interact differently with the column.
Software that simplifies workflows
Agilent Buffer Advisor Software simplifies your workflow by eliminating tedious and error-prone method development steps of buffer preparation, buffer blending, and pH scouting. Using the mixing principle of the Agilent 1260 Infinity Bio-inert Quaternary Pump, Buffer Advisor Software facilitates dynamic mixing of solvents from only four stock solutions, simplifying the bioanalysis workflow and significantly reducing the time required for buffer preparation. In addition, buffers are prepared more accurately, which makes for more robust method transfer to other laboratories.
Increasing challenges require better solutions
Agilent solutions enable innovative disease research, accelerate drug discovery, and deliver greater confidence throughout development and manufacturing. See how Agilent's comprehensive range of BioHPLC columns helps deliver the answers you need to bring effective therapeutics to market, in genomics research, automation, separation, and detection. Watch out, too, for the soon-to-be released Ion-Exchange Chromatography for Biomolecule Analysis: a “How to” Guide to learn how easy it is to integrate IEX methods into your workflow. Then review how Agilent’s comprehensive range of BioHPLC columns, and the broad range of Agilent solutions in genomics research, automation, separation, and detection technologies, helps deliver the answers you need to bring effective therapeutics to market.
For Research Use Only. Not for use in diagnostic procedures.
>> Update My Profile | Subscribe to Access Agilent | Article Directory
Figure 2.
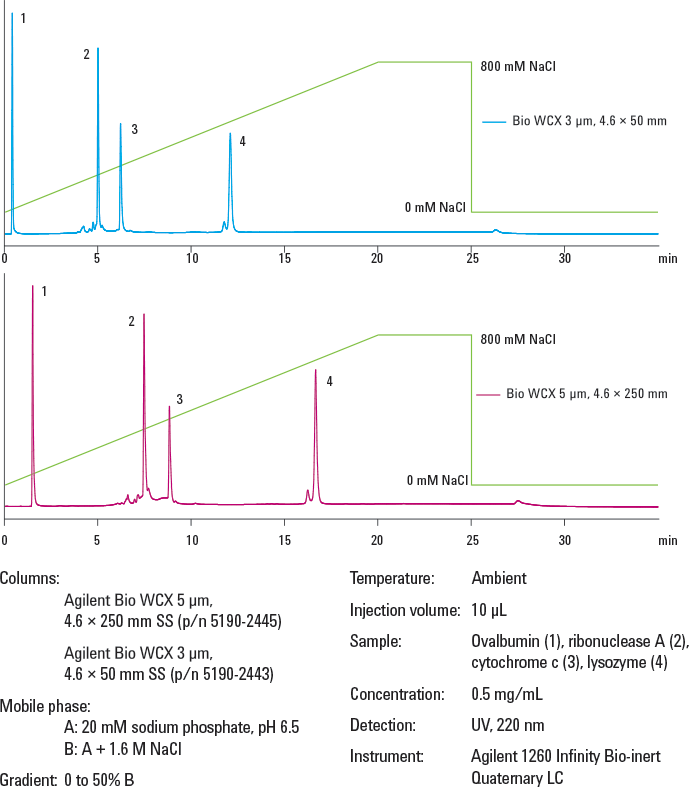
Protein separation on Agilent Bio WCX columns. Faster analysis times were achieved with smaller particle size and shorter column length – samples eluted from the longer column in 17 minutes but in only 12 minutes from the shorter column.